Abstract
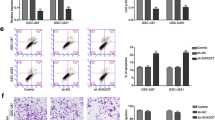
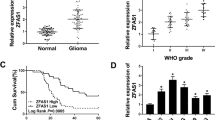
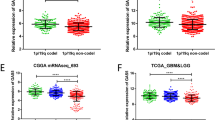
Glioma is one of the most common malignant tumors and shows a high metastasis rate and poor prognosis. Abnormal expression of long non-coding RNAs (lncRNAs) contributes to various human tumors including gliomas. This study aimed to investigate the regulatory role of the antisense RNA of growth arrest special 5 (GAS5-AS1), a novel lncRNA, in gliomas. Expression of GAS5-AS1 and microRNA-106b-5p (miR-106b-5p) in glioma tissues and cells was detected by quantitative reverse transcription PCR, northern blotting, or fluorescent in situ hybridization. Cell proliferation, migration, and invasion were analyzed by CCK-8 and Transwell assays. BALB/c nude mice were used to establish a glioma xenograft animal model by subcutaneous injection of U251 cells transfected with small interfering RNA targeting GAS5-AS1. A dual-luciferase reporter assay was conducted to confirm the targeting relationship between GAS5-AS1 and miR-106b-5p. GAS5-AS1 expression was downregulated in glioma tissues and cells, and upregulation of GAS5-AS1 expression inhibited glioma cell proliferation, migration, and invasion. GAS5-AS1 expression was correlated with the glioma tumor grade. In nude mice, upregulation of GAS5-AS1 markedly suppressed glioma tumor growth. GAS5-AS1 overexpression significantly increased the miR-106b-5p level in glioma cells, and GAS5-AS1 expression was negatively related to miR-106b-5p expression in glioma tissues. Overexpression of miR-106b-5p reversed the inhibitory effects of GAS5-AS1 upregulation on glioma cell proliferation and metastasis, while restoration of TUSC2 rescued the proliferation and invasion of glioma cells transfected with miR-106b-5p mimics. In summary, lncRNA GAS5-AS1 inhibited glioma proliferation, migration, and invasion by sponging miR-106b-5p and regulating the expression of TUSC2. Our results suggest GAS5-AS1 as a novel target for the treatment and prognosis prediction of gliomas.






Similar content being viewed by others
References
Werner MH, Phuphanich S, Lyman GH. The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer. 2015;76:1634–42.
Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2010;100:2235–41.
Valadez JG, Sarangi A, Lundberg CJ, Cooper MK (2014) primary orthotopic glioma xenografts recapitulate infiltrative growth and isocitrate dehydrogenase I mutation. J Vis Exp, pp e50865–e65.
Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9.
Zhang F, Zhang L, Zhang C. Long noncoding RNAs and tumorigenesis: genetic associations, molecular mechanisms, and therapeutic strategies. Tumour Biol. 2016;37:163–75.
Matjašič A, Glavač D (2015) Long Noncoding RNAs and Tumorigenesis
Tang J, Jiang R, Deng L, Zhang X, Sun B. Circulation long non-coding RNAs act as biomarkers for predicting tumorigenesis and metastasis in hepatocellular carcinoma. Oncotarget. 2015;6:4505–15.
Ricciuti B, Mencaroni C, Paglialunga L, et al. Long noncoding RNAs: new insights into non-small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33:18.
Li W, Li N, Shi K, Chen Q. Systematic review and meta-analysis of the utility of long non-coding RNA GAS5 as a diagnostic and prognostic cancer biomarker. Oncotarget. 2017;8:66414–25.
Wu Y, Lyu H, Liu H, Shi X, Song Y, Liu B. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci Rep. 2016;6:31093–193.
Abbastabar M, Sarfi M, Golestani A, Khalili E. lncRNA involvement in hepatocellular carcinoma metastasis and prognosis. EXCLI J. 2018;17:900–13.
Wu W, Lin Z, Zhuang Z, Liang X. Expression profile of mammalian microRNAs in endometrioid adenocarcinoma. Eur J Cancer Prev. 2009;18:50–5.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Yang M, Chen J, Su F, Yu B, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117.
Yu S, Qin X, Chen T, Zhou L, Feng J. MicroRNA-106b-5p regulates cisplatin chemosensitivity by targeting polycystic kidney disease-2 in non-small-cell lung cancer. Anticancer Drugs. 2017;28:852–60.
Ni S, Weiwei W, Midie X, et al. miR-106b-5p inhibits the invasion and metastasis of colorectal cancer by targeting CTSA. Oncotargets Therapy. 2018;11:3835–45.
Zheng L, Zhang Y, Liu Y, et al. MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J Transl Med. 2015;13:252.
Liu F, Gong J, Huang W, et al. MicroRNA-106b-5p boosts glioma tumorigensis by targeting multiple tumor suppressor genes. Oncogene. 2014;33:4813–22.
Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710.
Freije AW. Gene expression profiling of gliomas strongly predicts survival. Can Res. 2004;64:6503–10.
Park JY, Lee JE, Park JB, Yoo H, Lee S-H, Kim JH. Roles of long non-coding rnas on tumorigenesis and glioma development. Brain Tumor Res Treat. 2014;2:1–6.
Ma XL, Zhu WD, Tian LX et al (2017) Long non-coding RNA TUSC7 expression is independently predictive of outcome in glioma. Eur Rev Med Pharmacol Sci, pp 3605–3610.
Wang Y, Jing W, Ma W, Liang C, Chai H, Tu J. Down-regulation of long non-coding RNA GAS5-AS1 and its prognostic and diagnostic significance in hepatocellular carcinoma. Cancer Biomark Sect A Dis Markers. 2018;22:227–36.
Hu W, Gao A. The role of long non-coding RNAs in hematologic malignancies. Hereditas. 2015;37:1095.
Zhao X, Wang P, Liu J, et al. Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol Ther. 2015;23:1899–911.
Nama S, Muhuri M, Di Pascale F, et al. MicroRNA-138 is a prognostic biomarker for triple-negative breast cancer and promotes tumorigenesis via TUSC2 repression. Sci Rep. 2019;9:12718.
Xie HH, Huan WT, Han JQ, Ren WR, Yang LH. MicroRNA-663 facilitates the growth, migration and invasion of ovarian cancer cell by inhibiting TUSC2. Biol Res. 2019;52:18.
Xin J, Zhang XK, Xin DY, et al. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol Rep. 2015;34:868–76.
Funding
This work was supported by National Natural Science Foundation of China (81302181) and the Key Research and Development Program of Jiangsu Province (No. BE2019652).
Author information
Authors and Affiliations
Contributions
FL conceived and designed research; WH and YS performed experiments; BH analyzed data; QW interpreted results of experiments; BZ prepared figures; CQ drafted, edited, and revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of Nanjing University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Changzhou No. 2 People’s Hospital, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, W., Shi, Y., Han, B. et al. LncRNA GAS5-AS1 inhibits glioma proliferation, migration, and invasion via miR‐106b-5p/TUSC2 axis. Human Cell 33, 416–426 (2020). https://doi.org/10.1007/s13577-020-00331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00331-z