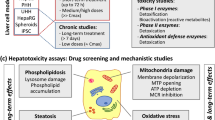
Abstract
HepaRG cells are widely used as an in vitro model to assess drug-induced hepatotoxicity. However, only few studies exist so far regarding their suitability to detect the effects of drugs requiring a preceding activation via the cytochrome P450 (CYP) system. A prototypic substance is the anti-tuberculosis agent INH, which is metabolized into N-acetylhydrazine, which then triggers hepatotoxicity. Therefore, the aim of the present study was to test if this effect can also be detected in HepaRG cells and if it can be counteracted by the known hepatoprotectant silibinin. For this purpose, differentiated HepaRG cells were treated with increasing concentrations of INH (0.1–100 mM) or 10 mM INH plus escalating concentrations of silibinin (1–100 µM). After 48 h of treatment, cell morphology and parameters indicating cell vitality, oxidative stress, and liver cell function were assessed. High concentrations of INH led to severe histopathological changes, reduced cell vitality and glutathione content, increased LDH and ASAT release into the medium, enhanced lipid peroxidation, and elevated cleaved caspase-3 expression. Additionally, glycogen depletion and reduced biotransformation capacity were seen at high INH concentrations, whereas at low concentrations an induction of biotransformation enzymes was noticed. Silibinin caused clear-cut protective effects, but with few parameters INH toxicity was even aggravated, most probably due to increased metabolization of INH into its toxic metabolite. In conclusion, HepaRG cells are excellently suited to evaluate the effects of substances requiring prior toxification via the CYP system, such as INH. They additionally enable the identification of complex substance interactions.






Similar content being viewed by others
Abbreviations
- ASAT:
-
Aspartate transaminase
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- CYP:
-
Cytochrome P450
- DMSO:
-
Dimethylsulfoxide
- ECOD:
-
Ethoxycoumarin-O-deethylation
- EROD:
-
Ethoxyresorufin-O-deethylation
- GSH:
-
(Reduced) glutathione
- GST:
-
Glutathione-S-transferase
- INH:
-
Isoniazid
- LDH:
-
Lactate dehydrogenase
- LPO:
-
Lipid peroxidation products
- NAT:
-
N-Acetyltransferase
- PHH:
-
Primary human hepatocytes
- PNPH:
-
p-Nitrophenol hydroxylase
References
Groeneberg DA, Grosse-Siestrup C, Fischer A. In vitro models to study hepatotoxicity. Toxicol Pathol. 2002;30:394–9.
Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106(Suppl 2):511–32.
Guillouzo A, Guguen-Guillouzo C. Evolving concepts in liver tissue modeling and implications for in vitro toxicology. Expert Opin Drug Metab Toxicol. 2008;4:1279–94.
Soldatow VY, LeCluyse EL, Griffith LG, Rusyn I. In vitro models for liver toxicity testing. Toxicol Res. 2013;2:23–39.
Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23.
Snawder JE, Lipscomb JC. Interindividual variance of cytochrome P450 forms in human hepatic microsomes: correlation of individual forms with xenobiotic metabolism and implications in risk assessment. Regul Toxicol Pharmacol. 2000;32:200–9.
LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–68.
Castell JV, Jover R, Martinez-Jimenez CP, Gomez-Lechon MJ. Hepatocyte cell lines: their use, scope and limitations in drug metabolism studies. Expert Opin Drug Metab Toxicol. 2006;2:183–212.
Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab Dispos. 2011;39:528–38.
Donato MT, Jover R, Gomez-Lechon MJ. Hepatic cell lines for drug hepatotoxicity testing: limitations and strategies to upgrade their metabolic competence by gene engineering. Curr Drug Metab. 2013;14:946–68.
Knasmuller S, Mersch-Sundermann V, Kevekordes S, Darroudi F, Huber WW, Hoelzl C, Bichler J, Majer BJ. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of the knowledge. Toxicology. 2004;198:315–28.
Aninat C, Piton A, Glaise D, Le Charpentier T, Langouet S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83.
Lubberstedt M, Muller-Vieira U, Mayer M, Biemel KM, Knospel F, Knobeloch D, Nussler AK, Gerlach JC, Zeilinger K. HepaRG human hepatic cell line utility as a surrogate for primary human hepatocytes in drug metabolism assessment in vitro. J Pharmacol Toxicol Methods. 2011;63:59–68.
Tomida T, Okamura H, Satsukawa M, Yokoi T. Multiparametric assay using HepaRG cells for predicting drug-induced liver injury. Toxicol Lett. 2015;236:16–24.
Wu Y, Geng X, Wang J, Miao Y, Lu Y, Li B. The HepaRG cell line, a superior in vitro modelt o L-02, HepG2 and hiHeps cell lines for assessing drug-induced liver injury. Cell Biol Toxicol. 2016;32:37–59.
Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, Drenou B, Gripon P, Kremsdorf D, Guguen-Guillouzo C, Corlu A. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology. 2007;45:957–67.
Parent R, Marion M-J, Furio L, Trepo C, Petit M-A. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology. 2004;126:1147–56.
Guillouzo A, Corlu A, Aninat C, Glaise D, Motel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73.
Gerets HHJ, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol. 2012;2012(28):69–87.
Kotani N, Maeda K, Debori Y, Camus S, Li R, Chesne C, Sugiyama Y. Expression and transport function of drug uptake transporters in differentiated HepaRG cells. Mol Pharm. 2012;9:3434–41.
Le Vee M, Noel G, Jouan E, Stieger B, Fardel O. Polarized expression of drug transporters in differentiated human hepatoma HepaRG cells. Toxicol In Vitro. 2013;27:1979–86.
Bachour-El Azzi P, Sharanek A, Burban A, Li R, Guevel RL, Abdel-Razzak Z, Stieger B, Guguen-Guillouzo C, Guillouzo A. Comparative localization and functional activity of the main hepatobiliary transporters in HepaRG cells and primary human hepatocytes. Toxicol Sci. 2015;145:157–68.
Kanebratt KP, Andersson TB. Evaluation of HepaRG cells as an in vitro model for human drug metabolism studies. Drug Metab Dispos. 2008;36:1444–52.
Kanebratt KP, Andersson TB. HepaRG cells as an in vitro model for evaluation of cytochrome P450 induction in humans. Drug Metab Dispos. 2008;36:137–45.
Turpeinen M, Tolonen A, Chesne C, Guillouzo A, Uusitalo J, Pelkonen O. Functional expression, inhibition and induction of CYP enzymes in HepaRG cells. Toxicol In Vitro. 2009;23:748–53.
Darnell M, Schreiter T, Zeilinger K, Urbaniak T, Söderdahl T, Rossberg I, Dillner B, Berg A-L, Gerlach JC, Andersson TB. Cytochrome P450-dependent metabolism in HepaRG cells cultured in a dynamic three-dimensional bioreactor. Drug Metab Dispos. 2011;39:1131–8.
Josse R, Aninat C, Glaise D, Dumont J, Fessard V, Morel F, Poul JM, Guguen-Guillouzo C, Guillouzo A. Long-term functional stability of human HepaRG hepatocytes and use for chronic toxicity and genotoxicity studies. Drug Metab Dispos. 2008;36:1111–8.
Antherieu S, Chesne C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, Guillouzo A. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38:516–25.
Savary CC, Jiang X, Aubry M, Josse R, Kopp-Schneider A, Hewitt P, Guillouzo A. Transcriptomic analysis of untreated and drug-treated differentiated HepaRG cells over a 2-week period. Toxicol In Vitro. 2015;30:27–35.
Klein S, Mueller D, Shevchenko V, Noor F. Long-term maintenance of HepaRG cells in serum-free conditions and application in a repeated dose study. J Appl Toxicol. 2014;34:1078–86.
Dumont J, Josse R, Lambert C, Antherieu S, Le Hegarat L, Aninat C, Robin MA, Guguen-Guillouzo C. Differential toxicity of heterocyclic aromatic amines and their mixture in metabolically competent HepaRG cells. Toxicol Appl Pharmacol. 2010;245:256–63.
McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–82.
Huang Y, Chern H, Su W, Wu J, Chang S, Chiang C, Chang F, Lee S. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–30.
Hassan HM, Guo H, Yousef BA, Luyong Z, Zhenzhou J. Hepatotoxicity mechanisms of isoniazid: a mini-review. J Appl Toxicol. 2015;35:1427–32.
Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WCM, van der Ven AJAM, Dekhuijzen R. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202.
Dixit N, Baboota S, Kohli K, Ahmad S, Ali J. Silymarin: a review of pharmacological aspects and bioavailability enhancement approaches. Indian J Pharmacol. 2007;39:172–9.
Vargas-Mendoza N, Madrigal-Santillan E, Morales-Gonzalez A, Esquivel-Soto J, Esquivel-Chirino C, Garcia-Luna y Gonzalez-Rubio M, Gayosso-de-Lucio JA, Morales-Gonzalez JA. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6:144–9.
Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4:204–47.
Schwab CE, Tuschl H. In vitro studies on the toxicity of isoniazid in different cell lines. Hum Exp Toxicol. 2003;22:607–15.
Shen C, Meng Q, Zhang G, Hu W. Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. Br J Pharmacol. 2008;153:784–91.
Singh M, Sasi P, Rai G, Gupta VH, Amarapurkar D, Wangika PP. Studies on toxicity of antitubercular drugs namely isoniazid, rifampicin, and pyrazinamide in an in vitro model of HepG2 cell line. Med Chem Res. 2011;20:1611–5.
Lee KK, Fujimoto K, Zhang C, Schwall CT, Alder NN, Pinkert CA, Krueger W, Rasmussen T, Boelsterli UA. Isoniazid-induced cell death is precipitated by underlying mitochondrial complex I dysfunction in mouse hepatocytes. Free Rad Biol Med. 2013;65:584–94.
Boojar MMA, Hassanipour M, Mehr ES, Boojar MMA, Dehpour AR. New aspects of silibinin stereoisomers and their 3-O-galloyl derivatives on cytotoxicity and ceramide metabolism in Hep G2 hepatocarcinoma cell line. Iran J Pharmaceut Res. 2016;15:412–33.
Ezhilarasan D, Evraerts J, Brice S, Buc-Calderon P, Karthikeyan S, Sokal E, Naji M. Silibinin inhibits proliferation and migration of human hepatic stellate LX-2 cells. J Clin Exp Hepatol. 2016;6:167–74.
Smolowitz RM, Hahn ME, Stegeman JJ. Immunohistochemical localization of cytochrome P-450IA1 induced by 3,3′,4,4′-tetrachlorobiphenyl and by 2,3,7,8-tetrachlorodibenzoafuran in liver and extrahepatic tissues of the teleost Stenotomus chrysops (scup). Drug Metab Dispos. 1991;19:113–23.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337–51.
Aitio A. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal Biochem. 1978;85:488–91.
Pohl RJ, Fouts JR. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem. 1980;107:150.
Chang TK, Crespi CL, Waxman DJ. Spectrophotometric analysis of human CYP2E1-catalyzed p-nitrophenol hydroxylation. Methods Mol Biol. 2006;320:127–31.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9.
Hinchman CA, Matsumoto H, Simmons TW, Ballatori N. Intrahepatic conversion of a glutathione conjugate to its mercapturic acid. Metabolism of 1-chloro-2,4-dinitrobenzene in isolated perfused rat and guinea pig livers. J Biol Chem. 1991;266:22179–85.
Klinger W, Muller D. The influence of age on the protein concentration in serum, liver and kidney of rats determined by various methods. Z Versuchstierk. 1974;16:149–53.
Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22:145–55.
Ostapowicz GM, Fontana R, Schiødt F, Larson A, Davern T, Han HS, McCashland T, Shakil A, Hay J, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM, U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54.
Leise MD, Poterucha JJ, Talwalkar JA. Drug induced liver injury. Mayo Clin Proc. 2014;89:95–106.
Zumla A, Chakaya J, Centis R, D’Ambrosio L, Mwaba P, Bates M, Kapata N, Nyirenda T, Chanda D, Mfinanga S, Hoelscher M, Maeurer M, Migliori GB. Tuberculosis treatment and management—an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir Med. 2015;3:220–34.
Sarma GR, Immanuel C, Kailasam S, Narayana AS, Venkatesan P. Rifampin-induced release of hydrazine from isoniazid. A possible cause of hepatitis during treatment of tuberculosis with regimens containing isoniazid and rifampin. Am Rev Respir Dis. 1986;133:1072–5.
Sodhi CP, Rana SV, Mehta SK, Vaiphei K, Attari S, Mehta S. Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug Chem Toxicol. 1997;20:255–69.
Tafazoli S, Mashregi M, O’Brien PJ. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol Appl Pharmacol. 2008;229:94–101.
Ryan DE, Ramanathan L, Iida S, Thomas PE, Haniu M, Shively JE, Lieber CS, Levin W. Characterization of a major form of rat hepatic microsomal cytochrome P-450 induced by isoniazid. J Biol Chem. 1985;260:6385–93.
Ueng TH, Ueng YF. Induction of cytochrome P-450-dependent monooxygenases by isoniazid in hamster liver, kidney and lung. J Formos Med Assoc. 1991;90:723–30.
Park KS, Sohn DH, Veech RL, Song BJ. Translational activation of ethanol-inducible cytochrome P450 (CYP2E1) by isoniazid. Eur J Pharmacol. 1993;248:7–14.
Zand R, Nelson SD, Slattery JT, Thummel KE, Kalhorn TF, Adams SP, Wright JM. Inhibition and induction of cytochrome P4502E1-catalyzed oxidation by isoniazid in humans. Clin Pharmacol Ther. 1993;54:142–9.
Malekinejad H, Rahmani F, Valivande-Azar S, Taheri-Broujerdi M, Bazargani-Gilani B. Long-term administration of silymarin augments proinflammatory mediators in the hippocampus of rats: evidence for antioxidant and pro-oxidant effects. Hum Exp Toxicol. 2012;31:921–30.
Prochazkova D, Bousova I, Wilhelmov N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–23.
Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J Pharmacol Exp Ther. 2010;332:1081–7.
Dvorak Z, Vrzal R, Ulrichova J. Silybin and dehydrosilybin inhibit cytochrome P450 1A1 catalytic activity: a study in human keratinocytes and human hepatoma cells. Cell Biol Toxicol. 2006;22:81–90.
Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett. 2010;291:120–9.
Lee CK, Choi JS. Effects of silibinin, inhibitor of CYP3A4 and P-glycoprotein in vitro, on the pharmacokinetics of paclitaxel after oral and intravenous administration in rats. Pharmacology. 2010;85:350–6.
Doehmer J, Weiss G, McGregor GP, Appel K. Assessment of a dry extract from milk thistle (Silybum marianum) for interference with human liver cytochrome-P450 activities. Toxicol In Vitro. 2011;25:21–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13577_2017_175_MOESM1_ESM.tif
Supplementary material 1 (TIFF 2139 kb) Supplemental Figure S1 INH induced cytotoxicity in undifferentaited and in differentiated HepaRG cells. Undifferentiated and differentiated HepaRG cells were either left untreated (control) or were treated for 48 h with 10 mM or 100 mM INH after which phase contrast microscopy was performed. Original magnification: × 100. Representative photomicrographs from 3 independent experiments with three independent batches of cells are shown. Arrows: hepatocyte-like cells; asterisks: cholangiocyte-like cells
13577_2017_175_MOESM2_ESM.tif
Supplementary material 2 (TIFF 2414 kb) Supplemental Figure S2 Induction of CYP enzyme activities by prototypical inducers in HepaRG cells. HepaRG cells were either left untreated or were treated for 24 h with 25 µM β-naphthoflavone (BNF) or with 100 µM phenobarbital (PB). The cells were then harvested and (A) ethoxyresorufin-O-deetylation (EROD) activity or (B) ethoxycoumarin-O-deethylation (ECOD) activity was measured. Data are given as mean ± standard error of the mean (SEM), n = 3 independent batches of cells. *: p ≤ 0.05 versus untreated cells (control) (Dunnett’s post hoc test)
13577_2017_175_MOESM3_ESM.tif
Supplementary material 3 (TIFF 5997 kb) Supplemental Figure S3 CYP enzyme activities in HepG2 cells, in HepaRG cells, and in human liver 9000 g supernatants. (A) Ethoxyresorufin-O-deetylation (EROD) activity, (B) ethoxycoumarin-O-deethylation (ECOD) acitivity, (C) glutathione-S-transferase activity using 1-chloro-2,4-dinitrobenzene as a substrate, and (F) p-nitrophenol hydroxylase (PNPH) activity. For preparation of human liver 9000 g supernatants, human liver samples were homogenized in 0.1 M sodium phosphate buffer pH 7.4 (1:3 w/v) and centrifuged at 9000 × g for 30 min. Data are given as mean ± standard error of the mean (SEM), n = 3 independent batches of cells or 3 different human liver 9000 g supernatants
13577_2017_175_MOESM4_ESM.tif
Supplementary material 4 (TIFF 8909 kb) Supplemental Figure S4 Influence of different concentrations of silibinin on different parameters for cytotoxicity in HepaRG cells. Differentiated HepaRG cells were incubated for 48 h with increasing concentrations (0 µM-100 µM) of silibinin after which (A) cell viability was quantified by means of the CCK-8 assay, (B) LDH leakage, (C) ASAT release into the medium was measured. Data are given as mean ± standard error of the mean (SEM), n = 8 (A) or n = 3 (B, C) independent batches of cells. *: p ≤ 0.05 versus untreated cells (control) (Dunnett’s post hoc test). (D, E) Silibinin-treated cells were embedded in paraffin and 4-µm-sections were prepared from the paraffin blocks. (D) Hematoxylin-eosin staining; original magnification: x630. (E) PAS staining; original magnification: x630. Representative photomicrographs from 3 independent experiments with three independent batches of cells are shown
13577_2017_175_MOESM5_ESM.tif
Supplementary material 5 (TIFF 5928 kb) Supplemental Figure S5 Influence of silibinin on oxidative state and apoptosis rate in HepaRG cells. Differentiated HepaRG cells were incubated for 48 h with increasing concentrations (0 µM-100 µM) of silibinin after which (A) protein content of the cell pellet, (B) the cellular content of reduced glutathione, and (C) the concentration of lipid peroxidation products were measured. Data are given as mean ± standard error of the mean (SEM), n = 6 (A) or n = 3 (B, C) independent batches of cells. *: p ≤ 0.05 versus untreated cells (control) (Dunnett’s post hoc test). (D) Silibinin-treated cells were embedded in paraffin and 4-µm-sections were prepared from the paraffin blocks and stained for cleaved caspase-3 expression. Immunohistochemistry, counterstaining with hematoxylin; original magnification: x630. Representative photomicrographs from 3 independent experiments with three independent batches of cells are shown
13577_2017_175_MOESM6_ESM.tif
Supplementary material 6 (TIFF 11069 kb) Supplemental Figure S6 Influence of silibinin on biotransformation capacity in HepaRG cells. Differentiated HepaRG cells were incubated for 48 h with increasing concentrations (0 µM-100 µM) of silibinin after which (A) ethoxyresorufin-O-deetylation (EROD) activity, (B) ethoxycoumarin-O-deethylation (ECOD) activity, (C) glutathione-S-transferase activity using 1-chloro-2,4-dinitrobenzene as a substrate, and (F) p-nitrophenol hydroxylase (PNPH) activity were measured. Data are given as mean ± standard error of the mean (SEM), n = 3 independent batches of cells. *: p ≤ 0.05 versus untreated cells (control) (Dunnett’s post hoc test). (D, E) Silibinin-treated cells were embedded in paraffin and 4-µm-sections were prepared from the paraffin blocks and stained for (D) CYP1A2, (E) CYP2E1 expression. Immunohistochemistry, counterstaining with hematoxylin; original magnification: x630. Representative photomicrographs from 3 independent experiments with three independent batches of cells are shown
Rights and permissions
About this article
Cite this article
Mann, A., Pelz, T., Rennert, K. et al. Evaluation of HepaRG cells for the assessment of indirect drug-induced hepatotoxicity using INH as a model substance. Human Cell 30, 267–278 (2017). https://doi.org/10.1007/s13577-017-0175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-017-0175-9