Abstract
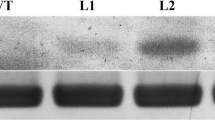
Due to the resistance of Artemia urmiana to salt stress, researchers have isolated and investigated Artemin, the most prevalent protein within the cyst of this aquatic species. In vitro studies have revealed Artemin's role as a molecular chaperone, effectively engaging with the hydrophobic surfaces of unfolded and/or partially folded proteins. In light of Artemin's established functional significance, its encoding gene has been successfully introduced into mammalian cells; however, no published research has elucidated its potential role within plant cells. In the current investigation, the artemin gene was successfully cloned into the pPZPY122 plant vector and subsequently introduced into Arabidopsis thaliana plants. The T3 homozygote transgenic plants (art) were then subjected to a series of environmental stresses, including heat, salt (NaCl) and drought (Mannitol). To assess the mutant's resilience to these stresses, their seed germination indices were evaluated. The art line demonstrated a higher degree of tolerance towards the abiotic stresses. A comparative analysis revealed that ascorbate peroxidase activity, catalase activity, and proline content exhibited significantly enhanced levels in some NaCl-treated art plants compared to their counterparts in Col-0. Regarding the expression of the genes in the SOS pathway, it was found that SOS1 is significantly upregulated under NaCl treatment in the art mutant. Conversely, under normal growth conditions, the morphology and growth of transgenics remained indistinguishable from those of wild-type plants.











Similar content being viewed by others
Abbreviations
- APX :
-
Ascorbate peroxidase
- art :
-
Artemin lines
- ART :
-
artemin Gene
- CAT :
-
Catalase
- Col-0:
-
Colombia-0
- Csp:
-
Cold shock proteins
- FGP :
-
Final germination percentage;
- GB :
-
Glycine betaine
- GI:
-
Germination index
- Gm :
-
Gentamicin
- GP :
-
Germination percentage
- GR:
-
Germination rate,
- MDG:
-
Mean daily germination,
- MS :
-
Murashige and Skoog
- P5CS :
-
Pyrroline-5-carboxylatesynthetase
- PI4P :
-
Phosphatidylinositol 4-phosphate
- sHSP :
-
Small heat shock proteins
- SOD :
-
Superoxide dismutase
- SOS :
-
Salt overly sensitive
References
Ajithkumar IP, Panneerselvam R (2014) ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. under drought stress. Cell Biochem Biophys 68:587–595
Almeida DM, Oliveira MM, Saibo NJ (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol 40:326–345
Awasthi JP et al (2017) Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 12(4):e0176357
Baltanás FC, García-Navas R, Santos E (2021) SOS2 comes to the fore: differential functionalities in physiology and pathology. Int J Mol Sci 22(12):6613
Begum N, Wang L, Ahmad H, Akhtar K, Roy R, Khan MI et al (2022) Coinoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb Ecol 83:971–988. https://doi.org/10.1007/s00248-021-01815-7
Brindha C, Vasantha S, Raja AK, Tayade AS (2021) Characterization of the salt overly sensitive pathway genes in sugarcane under salinity stress. Physiol Plantarum 171(4):677–687
Buchanan BB, Gruissem W, Jones RL (2015) Biochemistry and molecular biology of plants. John wiley & sons
Castiglioni P et al (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 147(2):446–455
Cattivelli L et al (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Res 105(1–2):1–14
Chen T, Villeneuve TS, Garant KA, Amons R, MacRae TH (2007) Functional characterization of artemin, a ferritin homolog synthesized in Artemia embryos during encystment and diapause. FEBS J 274(4):1093–1101
de Dios AJ (2019) A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol 23:101136
Díaz P, Monza J, Márquez A (2005) Drought and saline stress. Lotus japonicus handbook, 978–1–4020–3735–1, Springer, Dordrecht, pp 39–50
Du B, Nian H, Zhang Z, Yang C (2010) Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminum tolerance. Acta Physiol Plant 32:883–890
Ellis RH, Hong TD, Roberts EH (1981) The influence of desiccation on cassava seed germination and longevity. Ann Bot-London 47(1):173-175
Fageria N, Moreira A (2011) The role of mineral nutrition on root growth of crop plants. Adv Agron 110:251–331
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55(396):307–319. https://doi.org/10.1093/jxb/erh003
Gaber A et al (2006) Glutathione peroxidase-like protein of Synechocystis PCC 6803 confers tolerance to oxidative and environmental stresses in transgenic Arabidopsis. Physio Plantarum 128(2):251–262
Gelvin SB (2006) Agrobacterium virulence gene induction. Agrobacterium protocols: pp 77–85
Grover A, Singh A, Blumwald E (2011) Transgenic strategies toward the development of salt-tolerant plants, In Agricultural salinity assessment and management. p 235–274
Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25:989–994
Hao S, Wang Y, Yan Y, Liu Y, Wang J, Chen S (2021) A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulture 7(6):132
Harris D, Tripathi R, Joshi A (2002) On-farm seed priming to improve crop establishment and yield in dry direct-seeded rice. Direct seeding: Research Strategies and Opportunities, International Research Institute, Manila, Philippines:231–240.
Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51(1):463–499
Hnilickova H, Kraus K, Vachova P, Hnilicka F (2021) Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 10(5):845
Hu Y, Bojikova-Fournier S, King AM, MacRae TH (2011) The structural stability and chaperone activity of artemin, a ferritin homologue from diapause-destined Artemia embryos, depend on different cysteine residues. Cell Stress Chaperones 16:133–141
Hunter EA, Glasbey CA, Naylor RE (1984) The analysis of data from germination tests. J Agr Sci-Cambridge 102(1):207-213
Im YJ, Ji M, Lee A, Killens R, Grunden AM, Boss WF (2009) Expression of Pyrococcus furiosus superoxide reductase in Arabidopsis enhances heat tolerance. Plant Physiol 151(2):893–904
Ivanyi-Nagy R, Davidovic L, Khandjian E, Darlix J-L (2005) Disordered RNA chaperone proteins: from functions to disease. Cel Mol Life Sciences 62:1409–1417
Jacob P, Hirt H, Bendahmane A (2017) The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J 15(4):405–414
Jan S, Parray J (2016) Approaches to Heavy Metal Tolerance in Plants. Springer, Singapore, p 9789811016929
Jeong JS et al (2013) OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol J 11(1):101–114
Kareska S (2010) Factors affecting hydrogen peroxidase activity. Essai 7(1):27
Katsuya-Gaviria K, Paris G, Dendooven T, Bandyra KJ (2022) Bacterial RNA chaperones and chaperone-like riboregulators: behind the scenes of RNA-mediated regulation of cellular metabolism. RNA Biol 19(1):419–436
Kaya MD, Okçu G, Atak M, Cıkılı Y, Kolsarıcı Ö (2006) Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur J Agron 24(4):291–295
Khosravi Z, Khalili MAN, Moradi S, Sajedi RH, Zeinoddini M (2018) The molecular chaperone artemin efficiently blocks fibrillization of TAU protein in vitro. Cell Journal (yakhteh) 19(4):569
Kotowski F (1926) Temperature relations to germination of vegetable seed. P Am Soc Hortic Sci 23:176–184
Kundrátová K, Bartas M, Pečinka P, Hejna O, Rychlá A, Čurn V, Červeň J. (2021) Transcriptomic and proteomic analysis of drought stress response in opium poppy plants during the first week of germination. Plants. 10;10(9):1878
Li J, Wang S, Yu J, Wang L, Zhou S (2013) A modified CTAB protocol for plant DNA extraction. Chinese Bulletin of Botany 48(1):72
Ludwiczak A, Osiak M, Cárdenas-Pérez S, Lubińska-Mielińska S, Piernik A (2021) Osmotic stress or ionic composition: which affects the early growth of crop species more? Agronomy 11(3):435
Ma B, Gao L, Zhang H, Cui J, Shen Z (2012) Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep 31:687–696
Ma L, Liu X, Lv W, Yang Y (2022) Molecular mechanisms of plant responses to salt stress. Front Plant Sci 13:934877
MacRae TH (2003) Molecular chaperones, stress resistance and development in Artemia franciscana. In: Seminars in cell & developmental biology,. vol 14. Elsevier, pp 251–258
Morales M, Munné-Bosch S (2019) Malondialdehyde: Facts and artifacts. Plant Physiol 180(3):1246–1250
Motulsky H (2014) Intuitive biostatistics: a nonmathematical guide to statistical thinking. Oxford University Press, Oxford, USA
Orlovsky, N., Japakova, U., Zhang, H., & Volis, S. (2016). Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L.(Chenopodiaceae). Plant diversity, 38(4), 183–189
Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B, Zhang WK, Zhang JS, Chen SY (2010) Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. The Plant J 62(2):316–329
Park HJ, Kim W-Y, Yun D-J (2016) A new insight of salt stress signaling in plant. Mol Cells 39(6):447
Piao HL, Xuan YH, Park SH, Je BI, Park SJ, Park SH, Kim CM, Huang J, Wang GK, Kim MJ & Kang SM (2010). OsCIPK31, a CBL-interacting protein kinase is involved in germination and seedling growth under abiotic stress conditions in rice plants. Mol Cells 30:19–27
Piri R, Moradi A, Balouchi H, Salehi A (2019) Improvement of cumin (Cuminum cyminum) seed performance under drought stress by seed coating and biopriming. Sci Hortic 257:108667
Priya, P., Patil, M., Pandey, P., Singh, A., Babu, V. S., & Senthil‐Kumar, M. (2023). Stress combinations and their interactions in plants database: a one‐stop resource on combined stress responses in plants. The Plant Journal
Qiu Z, MacRae TH (2008) ArHsp22, a developmentally regulated small heat shock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J 275(14):3556–3566
Queiroz MS et al (2019) Drought stresses on seed germination and early growth of maize and sorghum. J Agric Sci 11(2):310
Team, R. D. C. (2010). R: A language and environment for statistical computing. (No Title).
Rosero A, Granda L, Berdugo-Cely JA, Šamajová O, Šamaj J, Cerkal R (2020) A dual strategy of breeding for drought tolerance and introducing drought-tolerant, underutilized crops into production systems to enhance their resilience to water deficiency. Plants 9(10):1263
Ruan S, Xue Q, Tylkowska K (2002) The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci Technol 30(1):61–67
Rueden CT et al (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18:1–26
Saadaoui W, et al. (2023) Effects of drought stress induced by D-Mannitol on the germination and early seedling growth traits, physiological parameters and phytochemicals content of Tunisian squash (Cucurbita maximaDuch.) landraces. Front Plant Sci 14
Shahangian SS, Rasti B, Sajedi RH, Khodarahmi R, Taghdir M, Ranjbar B (2011) Artemin as an efficient molecular chaperone. The Protein J 30:549–557
Sharma A et al (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9(7):285
Silva-Correia J, Freitas S, Tavares RM, Lino-Neto T, Azevedo H (2014) Phenotypic analysis of the Arabidopsis heat stress response during germination and early seedling development. Plant Methods 10(1):1–11
Smith-Moore S et al (2018) Adeno-associated virus Rep proteins antagonize phosphatase PP1 to counteract KAP1 repression of the latent viral genome. P Natl Acad Sci USA 115(15):E3529–E3538. https://doi.org/10.1073/pnas.1721883115
Sofi P, Djanaguiraman M, Siddique K, Prasad P (2018) Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Indian J Plant Physi 23:796–809
The World Map of Salt Affected Soil FAO, UNITED NATIONS (2023) In. https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/global-map-of-salt-affected-soils/en/
Tych KM et al (2015) Life in extreme environments: single molecule force spectroscopy as a tool to explore proteins from extremophilic organisms. Biochem Soc T 43(2):179–185
Uzilday B, Turkan I, Sekmen AH, Ozgur R, Karakaya H (2012) Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci 182:59–70
Vanderauwera S et al (2012) AtWRKY15 perturbation abolishes the mitochondrial stress response that steers osmotic stress tolerance in Arabidopsis. P Natl Acad Sci USA 109(49):20113–20118
Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127(2):426–435
Wang, F. (2021). Semi-quantitative RT-PCR: An effective method to explore the regulation of gene transcription level affected by environmental pollutants. In Environmental Toxicology and Toxicogenomics: Principles, Methods, and Applications (pp. 95–103). New York, NY: Springer US.
Warner A, Brunet R, MacRae TH, Clegg J (2004) Artemin is an RNA-binding protein with high thermal stability and potential RNA chaperone activity. Arch Biochem Biophysics 424(2):189–200
Xue Y, Peng R, Xiong A, Li X, Zha D, Yao Q (2010) Over-expression of heat shock protein gene hsp26 in Arabidopsis thaliana enhances heat tolerance. Biol Plantarum 54:105–111
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125(1):199–208
Yang R, Yu G, Li H, Li X, Mu C (2020) Overexpression of small heat shock protein LimHSP16. 45 in Arabidopsis hsp17. 6II mutant enhances tolerance to abiotic stresses. Russ J Plant Physl 67:231–241
Zeid I, Shedeed Z (2006) Response of alfalfa to putrescine treatment under drought stress. Biol Plantarum 50:635–640
Zhang X, Henriques R, Lin S-S, Niu Q-W, Chua N-H (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1(2):641–646
Zhang H, Zhu J, Gong Z, Zhu J-K (2022) Abiotic stress responses in plants. Nat Rev Genetics 23(2):104–119
Zhang Y, Xu J, Li R, Ge Y, Li Y, Li R (2023) Plants’ response to abiotic stress: Mechanisms and strategies. International Journal of Molecular Sciences. Jun 30; 24(13):10915.
Acknowledgements
This work is based upon research funded by Iran National Science Foundation (INSF) under project number 95831962.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poormohammad, Z., Shahrokhi, S., Abedi, A. et al. Enhanced drought and salt tolerance of Arabidopsis thaliana by ectopic expression of the molecular chaperone artemin from Artemia urmiana. J. Plant Biochem. Biotechnol. (2024). https://doi.org/10.1007/s13562-024-00877-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13562-024-00877-1