Abstract
Alopecia areata (AA) is a complex autoimmune disease manifesting as a chronic inflammatory disease characterized by non-scarring patches of hair loss over the face, scalp, and body. Several treatments have been proposed for AA, but none are curative nor achieve a state of remission. The present consensus statement aims to present the evidence- and experience-based recommendations on the diagnosis and management of AA in Saudi Arabia. The Ministry of Health in Saudi Arabia has opted to initiate a meeting of a multidisciplinary group to discuss and concede on this topic. Eight dermatology experts and clinical pharmacists convened in eight consensus meetings. All content presented in this document was agreed upon by this working group, including diagnosis and severity assessment, prognostic indicators, and therapeutic options for AA. Special consideration was given to special patient populations including pediatric patients and patients with less frequent presentations of AA. Updates of the current recommendations will take place as new evidence evolves in the treatment of AA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The management of Alopecia Areata (AA) is influenced by various issues and can be approached differently by healthcare providers in their daily clinical practice. |
Numerous drugs and treatment methods have been proposed for AA, but none are curative. Currently available treatment options produce variable clinical outcomes, and none induce and sustain remission. |
This paper aims to offer current guidance, drawn from the best available evidence and the insights of expert dermatologists, for dermatologists and other healthcare professionals in Saudi Arabia who are responsible for managing patients with AA. |
The consensus development process was undertaken by a multidisciplinary group of eight experts, including six dermatologists and two clinical pharmacists. |
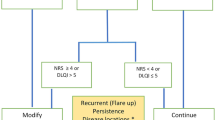
The success of AA treatment is achieved by meeting any or all of the following at week 24: Severity of Alopecia Tool (SALT) 75 reduction, SALT score 20 or less, achieving the Dermatology Life Quality Index (DLQI) of 5 or less. For Janus kinase inhibitors (JAKi), success of treatment should be reached maximum by week 36. |
Introduction
Background and Definition
Alopecia areata (AA) is a chronic inflammatory disease characterized by non-scarring hair loss with the preservation of the hair follicle [1]. It is a complex auto-immune disorder mediated by a local surge of interferon gamma that collapses the hair follicle immune privilege and elicits an autoimmune response targeting exposed hair follicle autoantigens by the activation of autoreactive cytotoxic CD8+NKG2D+T cells [2,3,4,5,6]. Relapse rates in AA are high irrespective of therapy, and even with corticosteroid therapy relapse rates range between 33% and 75% [2, 7]. AA may present in both sexes and at any age, but the peak incidence is between 20 and 50 years of age [8, 9]. The prevalence of AA in Saudi Arabia was found to be between 2.3% and 5.2% in recent studies [10, 11], a prevalence higher than that in Western countries [11]. Patients in the Saudi population were also observed to present at younger ages [10, 11].
Purpose and Aim
The management of AA is influenced by various issues and can be approached differently by healthcare providers in their daily clinical practice. Numerous drugs and treatment methods have been proposed for AA, but none are curative. Currently available treatment options produce variable clinical outcomes, and none induce and sustain remission. The current consensus was developed to provide recommendations based on the best available evidence and the experience of expert dermatologists. It is intended to benefit general dermatologists and other healthcare providers who manage patients with AA in Saudi Arabia.
Scope
Topics reviewed in this document include the clinical and diagnostic criteria of AA, the assessment of disease severity, evaluation of associated comorbidities, and the various treatment options available, ranging from conservative management to systemic treatments, along with a treatment algorithm developed for clinicians to refer to as needed. Special considerations for the management of the pediatric population with AA and treatment options in other special populations are also discussed.
Target Patient Population
This consensus statement is created for the optimal management and treatment of pediatric and adult patients with AA.
Targeted Audience/End-Users
General dermatologists and other healthcare providers who manage patients with AA in their clinical practice.
Methodology
A multidisciplinary group of eight experts, including six dermatologists and two clinical pharmacists were chosen based on their extensive clinical and research expertise in treating AA. The Saudi Ministry of Health provided the necessary oversight and support throughout the entire process. A total of eight consensus meetings were conducted, six of which were held virtually, and the remaining two were conducted in-person.
During the first meeting, the participating members defined the objectives, scope, aim, and target audience of the consensus statement. The work group identified three main topics of interest, which would serve as the sections for the review and consensus statement: (1) clinical features, diagnosis, and baseline investigations; (2) AA severity assessment and evaluation; and (3) management of AA and treatment options. Participating members were assigned to three workstreams based on their field of interest or expertise and they developed content for the consensus statement accordingly.
To guide the content development, the work group identified six international published consensus statements and disease management guidelines for AA. These documents served as the basis for the generation of this consensus, and they are as follows: the 2019 Italian Guidelines in diagnosis and treatment of alopecia areata, the 2019 Australian expert consensus statement, the 2020 Brazilian society of dermatology consensus on the treatment of alopecia areata, the Alopecia Areata Consensus of Experts (ACE) study parts I and II, published in 2020 and 2021, and the 2022 Japanese guidelines [3, 12,13,14,15,16]. The reference lists from the identified guideline and consensus statements were scrutinized to identify further literature and research that would guide the content of the consensus statement. A manual search of relevant literature was further performed as needed.
Prior to the third and fourth meetings, a preliminary draft was shared with the participating members. During the meetings, the participating members discussed the content and the formal consensus methodology. The nominal group technique was used to agree upon the recommendations included in this consensus document. All members were entitled to vote on the clinical recommendations presented. A statement was considered consented when at least 75% of the voting experts agreed. The strength of the recommendation was not expressed.
The document was then compiled and included all consensus recommendations agreed upon during the expert group meetings. The final document was later shared with the expert work group for further revision and feedback. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Clinical Features, Diagnosis, and Baseline Investigation
AA can manifest in several patterns. The most typical presentation is known as patchy alopecia which is characterized by the presence of single or multiple patches of localized hair loss. Other common presentations include alopecia totalis (AT) (complete loss of scalp hair) and alopecia universalis (AU) (total loss of body hair and scalp terminal hair) [14]. In rare cases, AA can present in other patterns, such as the ophiasis pattern (with band-like hair loss at the periphery of the occipital and temporal areas of the scalp), the ophiasis inversus-sisaipho pattern (with band-like hair loss in the frontoparietal area), and AA incognita pattern (with diffuse hair loss and thinning simulating androgenetic alopecia and telogen effluvium) [14]. Another very rare variant is peri nevoid AA, which is characterized by hair loss surrounding a pigmented nevus. Some patients present with AA signs that are localized to specific hairy regions outside the scalp, such as the beard, eyelashes, and eyebrows [14].
Patients with AA do not usually report other associated symptoms. Cutaneous dysesthesia may be reported and may precede or be associated with hair loss [14] and is a sign of active disease [15]. Patients might also present with scalp itching and tingling at the site of hair loss [15]. On physical exam, the skin underlying the affected patches usually appears normal and smooth and may rarely exhibit pink discoloration. White hairs may initially be spared in AA. In cases of extensive AA this leads to a phenomenon called canieties subita (sudden greying of the hair) [14]. Fine white vellus hairs may also be a sign of hair regrowth [14].
AA diagnosis is mainly clinical and is established through physical examination and trichoscopic findings [15]. This clinical diagnosis is supported by obtaining a detailed family history for autoimmune disorders (including but not limited to thyroid disorders, atopy, and celiac disease) [14]. The activity of hair loss can be determined by noting the presence of “exclamation point hairs” at the periphery of bald areas and the qualitative positive AA “hair pull test” [17]. Scalp biopsy is not recommended unless the diagnosis is not clear. The characteristic clinical features of AA include intact follicular ostia visualized either directly or through dermoscopy, positive pull test, complete loss of terminal hairs in at least one patch of hair loss, and the absence of signs of active follicular inflammation (such as perifollicular erythema, scaling, pustules, etc.) [17]. Yellow and/or black dots at the ostia on dermoscopy, trachyonychia, fine pitting of the nails, and 20-nail dystrophy are considered supportive features for the diagnosis of AA [17].
Assessment and Evaluation
Assessment Tools
Assessment of AA must be conducted in the initial patient encounter and during follow-up visits. There are multiple severity assessment tools to assess AA disease extent, these include the following: Severity of Alopecia Tool (SALT), SALT II, Alopecia Density and Extent (ALODEX), Alopecia Areata Progressive Index, and quality-of-life measures including Dermatology Life Quality Index (DLQI) and Alopecia Areata Symptom Impact Scale (AASIS). A more recent scoring scale that has been developed in 2022, the AA Scale which is designed specifically for use in clinical practice captures broader features of the disease [17, 18]. Eyelashes and eyebrows can be assessed using the Clinician-Reported Outcome (ClinRO) and Patient-Reported Outcome (PRO) [19]. Consensus was achieved on using the SALT score as a measure of disease severity in children and adults [17], and ClinRO for eyebrow and eyelash involvement assessment [19].
The SALT I scoring visual aid was first published in 1999 and updated in 2004. It provides a visual guide to estimate the area of terminal hair loss in each of the four quadrants of the scalp and then generate the percentage of scalp hair loss and SALT score by adding up the estimates from the four quadrants (Fig. 1). The SALT score allows clinicians to estimate the total area of the scalp with loss of terminal hair [20, 21].
Laboratory Evaluation
Laboratory evaluation is warranted upon clinical suspicion of an underlying disorder, and there is no set of tests that is to be routinely ordered for patients presenting with AA [15]. When there are clinical signs of a tinea capitis infection, fungal microscopy should be performed. Moreover, when patients present with clinical signs and symptoms that point towards another underlying etiology for hair loss, the clinician may opt to perform blood tests as needed (complete blood cell count; renal and liver function) and to screen for other diseases (such as autoimmune diseases, connective tissue disease, celiac disease, pernicious anemia, and diabetes). Viral serology maybe useful in patients who present with clinical symptoms suggestive of a triggered alopecia areata episode. Measuring early morning cortisol levels is not indicated in patients who believe that stress may have triggered their current episode. In preparation for the initiation of systemic treatment for AA patients, laboratory investigations must be done and are identical to those performed in other dermatological diseases [15].
Psychosomatic Evaluation
Identifying patients and families who need psychological support is a fundamental component of AA management. Children, adolescents, and adults with AA are at increased risk for psychiatric comorbidity, particularly mood and anxiety disorders. This is especially the case in patients with severe or recalcitrant disease. Parents of children affected by AA may also experience anxiety and depression [22,23,24].
Prognosis and Prognostic Indicators
AA is a chronic disease with an unpredictable course as previously indicated. Spontaneous remission of a single lesion is estimated at 70% in the first year, while around 7% progress to AT or AU [13]. Potential prognostic indicators for poor outcomes include the following:
-
1.
Childhood onset of AA, particularly those presenting at less than 6 years of age [25].
-
2.
Severity of disease at onset in adults (this is the most important negative prognostic indicator) [26].
-
3.
Duration of active disease; prognosis is worse when AA persists beyond 5 years (2% potential for hair regrowth) and disease can be irreversible if it persists for 10 years (However, this is not a contraindication for a trial of therapy, successful cases of hair regrowth have been seen in patients with active AA with disease duration exceeding 10 years) [15].
- 4.
-
5.
Involvement of the eyebrows, eyelashes, and non-scalp hair loss [15].
-
6.
Nail involvement; nail pitting and trachyonychia suggest an increased risk of developing AT/AU and worsen prognosis overall for AA [27].
-
7.
Prior and/or current atopic conditions, including atopic dermatitis, asthma, hay fever/allergic rhinitis, and/or allergic conjunctivitis [17].
-
8.
Trisomy 21 (Down syndrome); this syndrome is associated with an increased prevalence (9%) and severity of AA, it is estimated that 40% of patients with trisomy 21 will develop AT/AU [28].
The emergence of AA may also be influenced by systemic and immunological factors. Examples of such factors include personal history of diabetes mellitus, peptic ulcer, bilateral oophorectomy, hypertension, atherosclerosis, Grave’s disease, and myxedema. However, these are not confirmed to be poor prognostic factors [12, 15]. Type 1 diabetes and other autoimmune disorders such as celiac disease and rheumatoid arthritis have been shown to have a genetic association with AA, suggesting common molecular pathways in their pathogenesis [29, 30]. Family history of AA has not been proven to be a poor prognostic indicator [15].
Management of Alopecia Areata
Treatment Goals
Treatment of AA aims to restore hair density and improve the psychological wellbeing of impacted patients. Success of treatment is reached by meeting any or all of the following at week 24 (Fig. 2) [17]:
-
(A)
SALT 75 reduction
-
(B)
SALT score 20 or less
-
(C)
Achieving DLQI of 5 or less
For JAK inhibitors, success of treatment should be reached by week 36 [31, 32].
Conservative Management
Reassurance can be enough for patients with limited stable disease [12]. Cosmetic camouflage options can be suggested in some cases [13]. Cosmetic camouflages are available in different forms such as hair fibers, sprays, waxes, tattoos, trichopigmentation, and pigmented powders (can be applied on the scalp and eyebrow area). Patients can also use wigs, false eyelashes, and eyebrow prostheses [13, 14].
Non-Pharmacologic Treatment
Photochemotherapy
Psoralen plus ultraviolet A (PUVA) is not recommended as a treatment option for AA due to the risk of large cumulative doses of UVA in the long term, as AA is a chronic disease. This increases the risk of cutaneous malignancy [33]. UVA-1, which has greater penetration into the skin and is more effective, may be an alternative option in the treatment of AA [34].
Excimer Laser/Excimer Light
As previously mentioned, AA is considered a T-cell mediated autoimmune disorder. The 308-nm excimer laser and excimer light are thought to have immunosuppressive properties by inducing T-cell apoptosis. Therefore, they can be used as an alternative treatment and as an adjuvant therapy for acute/subacute resistant patches of AA [35]. The side effects include mild erythema, contact eczema, blistering, pruritus, and hyperpigmentation [36].
Intralesional Treatment
Intralesional Corticosteroid Therapy
The treatment algorithm for AA is shown in Fig. 3. Intralesional corticosteroids are the standard of therapy for patients with clinical evidence of active inflammation [13]. Positive pull test, black dots, and exclamation point hairs can be used as indicators of active inflammation [16]. The most commonly used corticosteroid is triamcinolone acetonide (TAC). The recommended dose for scalp patchy AA is 2.5 mg/ml at a higher volume of 8 ml per session [37].
For the procedure, TAC is diluted with saline then 0.05–0.1 ml (per injection) is injected into the dermis or upper part of the subcutaneous tissue. Injections are optimally spaced 0.5–1 cm apart, and a time interval of 4–6 weeks must separate the sessions [13]. Caution is required, especially near the frontal hairline as the risk of atrophy is higher [16]. Lidocaine can be mixed with TAC. However, some components of the lidocaine vehicle can increase the risk of flocculation of the drug, leading to more atrophy. This procedure is uncomfortable for patients; therefore, it is recommended to apply local anesthetics, cooling, or vibration to the area prior to the procedure to minimize discomfort. Needle-free devices are another option for this procedure. Treatment using TAC should be discontinued if no improvement is seen within 6 months [13]. Side effects of TAC include pain, bleeding, headaches, systemic absorption, dyspigmentation, reversible skin atrophy (lasting a few months) and, rarely, anaphylaxis [13].
Topical Treatment
Topical Corticosteroid Therapy
Topical corticosteroid therapy is the first-line topical treatment for scalp AA in children and adults who refuse intralesional corticosteroid therapy (Table 1) [14]. The recommended topical corticosteroid for scalp patchy AA in adults is ultrapotent topical corticosteroid, such as clobetasol propionate foam [37], cream, or ointment. This treatment is more effective under occlusion (it also results in more side effects in this situation) [13]. For children less than 10 years of age, a less potent corticosteroid, such as mometasone, is preferred [37]. These medications should be applied daily for a period of 6 weeks to 6 months. Complete regrowth of hair is the indication for treatment discontinuation [16]. Common side effects are folliculitis, acneiform eruption, atrophy, dyschromia, and telangiectasia. Adrenal suppression is less common [13]. After hair regrowth, tapering of potent topical corticosteroids should be considered based on each patient’s individual situation and the physician’s expertise and clinical judgement [14].
Minoxidil
The mechanism of action of minoxidil is still not known. Some theories regarding the mechanism of minoxidil propose that this drug leads to vasodilation, potassium channel opening, angiogenesis, and induction of follicular dermal papilla proliferation. Minoxidil acts by increasing the duration of the anagen phase of the hair cycle and, therefore, it can increase the length and thickness of the new hair [13]. In adults, 5% minoxidil applied once or twice daily is preferred [13].
The use of 5% minoxidil alone is insufficient in AA [13]. For this reason, minoxidil is used as an adjuvant therapeutic agent along with other treatments, and it may help maintain hair growth induced by other treatments [37]. Minoxidil is easy to use and has minimal side effects (mainly mild dermatitis and itching). Less than 5% of patients may grow unwanted hair [37].
Topical Immunotherapy (TIT)
TIT is used in extensive chronic AA. TIT includes diphenylcyclopropenone (DPCP) and squaric acid dibutyl-ester (SADBE). The mechanism of action is not fully understood. The proposed theory is that TIT allows the follicle to recover through induction of inflammatory process in a place other than the hair bulb [13]. TIT agents cause allergic contact dermatitis [14, 37].
DPCP is the most used TIT for treating AA. Its success rate ranges from 30% to 48%, with an overall response rate of 72.2% across all grades [13, 37, 38]. However, there is limited evidence supporting its effectiveness in cases of extensive resistant AA, and further well-designed randomized controlled trials are needed to establish its efficacy [39]. It is only available at compounding pharmacies as it should be formulated with acetone and kept in an amber bottle to protect it from light [13]. Patients need to undergo sensitization before starting the treatment. During the sensitizing phase, a small amount of 2% DPCP is applied with a cotton swab on a barely visible area of 4 cm diameter, such as the scalp area behind the ear [13]. Patients are instructed to cover the scalp and avoid light and water exposure in the first 48 h [13, 37]. One week after sensitization is performed, 0.001% DPCP is applied by the physician or nurse unilaterally with an increase in the concentration on a weekly basis until the effective (ideal) concentration for the patient is reached. This ideal concentration can be identified by the presence of mild tolerable dermatitis that lasts 36 h. Once there is response to the treatment on one side, both sides can be treated [37]. After hair regrowth, and during the weaning phase, the frequency of application is reduced to biweekly then monthly and finally the treatment is discontinued [13].
For patients who fail to respond to DPCP, therapy with SADBE may be attempted. It is important to note that treatment with these agents should not be discontinued abruptly, as this can increase the risk of relapse [37].
TIT sensitizers are contraindicated in pregnant women [37]. Common side effects include pigmentary changes and vitiligo particularly in patients with dark skin [14], severe eczema, blisters, lymph node enlargement, or flu-like symptoms [13].
Anthralin
Anthralin was historically used for psoriasis [14]. Anthralin reduces the inflammation of the hair follicle by diverting it from the bulb and causes irritant dermatitis. It has no systemic side effects; therefore, it can be used in children [13, 14]. Anthralin can be found in concentrations from 0.5% to 2% [14].
The drug is applied on daily basis for 30 min. It is applied on the affected area and up to 1 cm into the healthy area. Every 3 days the time of application is increased by 15 min until a maximum of 2 h is reached. The patient should wash the area thoroughly to remove the product. The initial response is expected in 3 months with complete response in 15 months [13]. The combination of anthralin and DPCP has been reported to be more effective than treatment with DPCP alone [14].
Another treatment method includes the application of anthralin (concentration 0.5% or 1% cream). The cream is applied for around 20–30 min daily. Contact time with the affected skin is to be increased by 10 min biweekly to a maximum time of 1 h or to the contact time that causes mild dermatitis. Alternatively, anthralin can be started at lower concentrations (0.1%). In this case, the patient would apply the cream to the scalp area for 10 to 20 min and then gradually increase contact time until having anthralin on the affected area overnight is tolerable [14].
The frequent application of anthralin can cause follicular ostia pigmentation. This can appear as black dots which can be differentiated by dermoscopy as brown pigmentation at the edge of the ostium, while the pigmentation in the black dots is in the center of the ostium [13].
Lack of pigmentation can be a sign of treatment failure. Possible causes for failure are inappropriate application, inadequate application time, or low-quality preparation [13]. The fact that anthralin can stain hair, skin, and clothes might limit its use in fair-haired patients [14].
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors can be used to treat scalp, eyebrow, or beard AA. They are not effective as a monotherapy and are usually used with other agents [16]. Topical tacrolimus is used for the treatment of AA, especially in the face region and in the pediatric population. It is applied twice daily for 6 months. Pimocrolimus cream (1%) efficacy in AA was not proved [16]. Overall, the data available on these medications is lacking and their routine use in AA is not recommended [13].
Systemic Treatment
Eligibility Criteria
There are no set universal indications for initiating systemic treatment for AA. However, possible indications for systemic treatment include [12, 16, 25]:
-
1.
Extensive disease (SALT score ≥ 50).
-
2.
SALT score < 50 and DLQI score ≥ 10.
-
3.
Rapid progression of hair loss to ≥ 50% within 4 months of disease onset.
-
4.
All lesions that have failed a 6-month trial of topical or serial intralesional corticosteroid.
-
5.
Patients with a large or multiple lesions of AA, that cannot be easily managed with intralesional therapy due to the discomfort associated with the number of injections required, and patients who experience dermal atrophy following intralesional corticosteroid or side effects following topical steroid use.
-
6.
Significant facial involvement (eyebrows or eyelashes).
Systemic Treatment Options
Systemic Corticosteroids
Prednisolone (prednisone) is the preferred choice when systemic corticosteroid therapy is to be instituted, and it is optimally administered daily (Table 2). Systemic corticosteroids are a suitable treatment option for severe AA in adolescents aged 13–18 years. Prednisolone is started initially at a dose of 0.4–0.6 mg/kg/day. It is eventually gradually tapered over a minimum 12-week duration for durable remission to be achieved. A tapering duration of more than 12 weeks may be needed to achieve durable remission in adult patients [16].
In adults with extensive AA, dexamethasone oral pulse can be used starting at 5 mg/day 2 days a week for at least 12 weeks. In one study, 63% of 30 patients had an excellent response with oral pulse therapy. Mild side effects were noted in a quarter of the patients [40].
Cyclosporine
Cyclosporine is an effective treatment option in adults with severe AA. It is optimally dosed at 3–5 mg/kg/day, with a treatment duration than does not exceed 6–12 months [16].
Methotrexate
Methotrexate can sometimes be used as a monotherapy in severe AA. In adults, dosing is 15–20 mg weekly. Methotrexate monotherapy is also an option in adolescents aged 13–18 years with severe cases of AA. In the latter patient population, the target dose is 0.4 mg/kg/week [16].
Azathioprine
Azathioprine is another treatment option for AA. This drug is usually started at a low dose of 0.5–1 mg/kg daily to reduce the risk of gastrointestinal upset. It is gradually titrated every 4–6 weeks, reaching a maximum dosing rate of 2–3 mg/kg and this titration is done based on the response recorded and patient tolerance to the drug [12].
Sulfasalazine
Sulfasalazine can be considered for the therapy of persistent AA [41]. This drug is initially dosed at 0.5 g twice daily for 1 month, then 1 g twice daily for 1 month, and eventually the dose reaches 1.5 g twice daily for 4 months. In one study, the relapse rate was 45.5%, and 32% of patients suffered from adverse effects (gastrointestinal distress, rash, headache, and laboratory abnormalities) [42, 43].
Due to lower efficacy of sulfasalazine and azathioprine, there is group consensus that these medications should not be used as first line by the treating physician and are to be used only as an alternative therapy [43, 44].
Janus Kinase (JAK) Inhibitors
JAK inhibitors are small-molecule drugs with a few currently in the pipeline for treatment of moderate to severe AA [45].
Options for JAK inhibitor treatment in AA include the following:
-
1.
Baricitinib (JAK1/2 inhibitor), which has recently been approved by the SFDA for treatment of severe AA in adults, dose 2–4 mg orally once daily.
-
2.
Ritlecitinib (JAK3 selective inhibitor), which has recently been approved by the FDA for individuals aged 12 years and older dosed at 30 mg or 50 mg once daily [46].
-
3.
Tofacitinib (JAK1/3 inhibitor), dosed at 5 mg orally twice daily to 10 mg twice a day in severe alopecia areata [47,48,49].
-
4.
Ruxolitinib, (JAK1/2 inhibitor), dosed at 20 mg orally twice daily. In an open-label pilot study, 75% of patients achieved at least 50% hair regrowth after 3–6 months of therapy [50].
Side effects of JAK inhibitors include infections, viral reactivation, dyslipidemia, transaminase changes, leukopenia, headache, gastrointestinal complaints, thromboembolic events in high-risk patients, fatigue and weight gain, and acne [50,51,52]. Long-term safety data is still limited [53].
Generally, the response to the treatment with JAK inhibitors does not correlate with disease severity nor duration, and there are no predictors of response up to this date [53].
Combination of Systemic Agents
Methotrexate (10–25 mg per week) can be useful when used in association with systemic steroids (clinical response rate of 63%) [54]. The combination of cyclosporine (2.5–5 mg/kg/day) with low-dose corticosteroids (methyl prednisone started as 20 mg/day then subsequently tapered to 2 mg/day) has adequate efficacy and minimal toxicity for the treatment of severe AA [55]. Tofacitinib 5 mg twice daily can be combined with systemic steroids in resistant cases of AA [56]. Moreover, the combination of tofacitinib 5 mg twice daily and low-dose oral minoxidil (2.5 mg once daily for women and 2.5 mg twice a day for men) has been shown to produce positive outcomes, with a low incidence of adverse effects [57]. The treating physician may consider a similar combination with baricitinib; however, further evidence is necessary to determine the best specific combination.
Finally, simvastatin (40 mg) can be combined with ezetimibe (10 mg) orally and administered daily for 3 months as an alternative treatment for recalcitrant AA [58], but data on this treatment option is still limited and controversial [59].
Treatment in Special Situations
AA in Children
In children with AA, topical corticosteroids, preferably of medium to high potency (mometasone 1% to clobetasol 0.05%), are used as the first therapeutic option. When there is contraindication or lack of response to topical corticosteroids, anthralin, DPCP, or minoxidil may be used [13].
The combination of two or all three of these therapeutics is considered the second-line management. Systemic therapy is considered a third-line management option in children below the age of 12 years. In the absence of data confirming that systemic therapy reduces the risk of subsequent development of AT or AU, there is no consensus on the use of systemic therapy in children below the age of 12 years [12]. A decision can be taken by an experienced dermatologist on a case-by-case basis.
AA in the Beard Area
Topical or intralesional corticosteroid therapy are the treatments of choice for AA affecting the beard. However, there is a higher risk of atrophy associated with the use of corticosteroids in this area. Triamcinolone 2.5 mg/ml is the most suitable concentration for this area. Topical minoxidil and anthralin may also be considered [13]. There is no consensus on the usage of calcineurin inhibitors for AA in the beard.
AA in the Eyelashes and Eyebrows
With respect to eyelash hypotrichosis, the treatment of choice consists of analogs of prostaglandin F2a (latanoprost or bimatoprost) in solution used once daily. This treatment should be used for a minimum of 12 months to be able to assess its response. It is important to note that there is a risk of eyelid hyperpigmentation and darkening of the iris with this treatment option [12,13,14].
With respect to AA in the eyebrows, treatment options include topical corticosteroid of medium potency in cream or intralesional triamcinolone (2.5 mg/ml). Alternatively, 0.03% bimatoprost or topical minoxidil can be used [13]. Systemic treatment may be considered after failure of topical and intralesional modalities if a patient reaches a ClinRo score of 2 or more.
Follow-Up After Treatment Initiation
The period between follow-up visits is dependent on the treatment regimen used. Patients on DPCP should be assessed weekly or biweekly, and those treated with intralesional corticosteroids need to be followed up every 4–6 weeks, while patients using topical treatment are usually assessed every 2–3 months [13].
Patients treated with systemic corticosteroid agents should be weaned over a duration of at least 3 months. Patients on steroid-sparing agents should continue treatment for 3–6 months after complete remission, and tapering can be attempted during that period. The process of weaning the medication helps in decreasing the risk of relapse. Nevertheless, relapse can occur during weaning. In the case of patients who are taking an adequate dosage of systemic agents who develop major relapses (multiple new patches), treatment should be discontinued, and other treatment options considered. Relapses can be treated by dose escalation, the use of intralesional triamcinolone acetonide, or a short course of systemic corticosteroids [12].
Response to treatment should be assessed clinically through history taking, assessing hair-shedding severity, obtaining serial photography of the affected areas, measuring the SALT score, and measuring the quality of life indexes [12]. Dermoscopic photos of the affected areas are recommended as well [16]. These assessment tools are utilized to correlate the response observed in the last visit to the situation in the initial visit (baseline). A SALT reduction of 75 (75% improvement in SALT) is an appropriate goal for a successful treatment regimen. However, increased hair shedding, positive pull test, especially at the edge of the patches, as well as exclamation mark hairs and black dots on dermoscopy, are indicators of active disease [12].
Conclusion
AA is a condition that impacts patients of all ages and can present in many ways. It is commonly encountered by general dermatologists and other healthcare providers. AA is a psychologically distressing disease and successful management is key to improve the quality of life of those affected. An adequate clinical assessment and evaluation, and an understanding of the treatment options available, are essential for a healthcare provider to manage this disease. This document serves as a guide for clinical practitioners to refer to and take the best approach in treating their patients with AA. An individualized plan should always be considered for patients with AA to achieve the best outcomes possible.
References
Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42(4):549–66 (quiz 567–70).
Kurosawa M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology. 2006;212(4):361–5.
Fukuyama M, Ito T, Ohyama M. Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J Dermatol. 2022;49(1):19–36.
Ito T, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Investig Dermatol. 2008;128(5):1196–206.
Ito T, et al. Collapse and restoration of MHC class-I-dependent immune privilege: exploiting the human hair follicle as a model. Am J Pathol. 2004;164(2):623–34.
Xing L, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–9.
Jahn-Bassler K, et al. Sequential high- and low-dose systemic corticosteroid therapy for severe childhood alopecia areata. J Dtsch Dermatol Ges. 2017;15(1):42–7.
Starace M, et al. Female androgenetic alopecia: an update on diagnosis and management. Am J Clin Dermatol. 2020;21(1):69–84.
Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol. 1996;35(1):22–7.
Alshahrani AA, et al. Prevalence and clinical characteristics of alopecia areata at a tertiary care center in Saudi Arabia. Dermatol Res Pract. 2020;2020: 7194270.
Al-Ajlan A, et al. Prevalence of alopecia areata in Saudi Arabia: cross-sectional descriptive study. Cureus. 2020;12(9): e10347.
Cranwell WC, et al. Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol. 2019;60(2):163–70.
Ramos PM, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(Suppl 1):39–52.
Rossi A, et al. Italian guidelines in diagnosis and treatment of alopecia areata. G Ital Dermatol Venereol. 2019;154:609–23.
Meah N, et al. The Alopecia Areata Consensus of Experts (ACE) study part II: results of an international expert opinion on diagnosis and laboratory evaluation for alopecia areata. J Am Acad Dermatol. 2021;84(6):1594–601.
Meah N, et al. The Alopecia Areata Consensus of Experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83(1):123–30.
Olsen EA, et al. Objective outcome measures: collecting meaningful data on alopecia areata. J Am Acad Dermatol. 2018;79(3):470-478.e3.
King BA, et al. Development of the alopecia areata scale for clinical use: results of an academic-industry collaborative effort. J Am Acad Dermatol. 2022;86(2):359–64.
Wyrwich KW, et al. Development of clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) measures for eyebrow, eyelash and nail assessment in alopecia areata. Am J Clin Dermatol. 2020;21(5):725–32.
Olsen E, et al. Alopecia areata investigational assessment guidelines. National Alopecia Areata Foundation. J Am Acad Dermatol. 1999;40(2 Pt 1):242–6.
Olsen EA, et al. Alopecia areata investigational assessment guidelines—Part II. National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51(3):440–7.
Ruiz-Doblado S, Carrizosa A, García-Hernández MJ. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42(6):434–7.
Koo JY, et al. Alopecia areata and increased prevalence of psychiatric disorders. Int J Dermatol. 1994;33(12):849–50.
Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47(11):1118–20.
Ikeda T. A new classification of alopecia areata. Dermatologica. 1965;131(6):421–45.
van der Steen PH, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2 Pt 1):227–30.
Tosti A, et al. Trachyonychia associated with alopecia areata: a clinical and pathologic study. J Am Acad Dermatol. 1991;25(2 Pt 1):266–70.
Du Vivier A, Munro DD. Alopecia areata, autoimmunity, and Down’s syndrome. Br Med J. 1975;1(5951):191–2.
Petukhova L, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466(7302):113–7.
Betz RC, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. 2015;6:5966.
King B, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–99.
King B, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol. 2021;85(4):847–53.
van der Schaar WW, Sillevis Smith JH. An evaluation of PUVA-therapy for alopecia areata. Dermatologica. 1984;168(5):250–2.
Herz-Ruelas ME, et al. Escalating dosimetry of UVA-1 in the treatment of alopecia areata. Int J Dermatol. 2017;56(6):653–9.
Ohtsuki A, et al. 308-nm excimer lamp for the treatment of alopecia areata: clinical trial on 16 cases. Indian J Dermatol. 2013;58(4):326.
Mlacker S, et al. A review on laser and light-based therapies for alopecia areata. J Cosmet Laser Ther. 2017;19(2):93–9.
Strazzulla LC, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1–12.
Lamb RC, Young D, Holmes S. Retrospective review of diphencyprone in the treatment of alopecia areata. Clin Exp Dermatol. 2016;41(4):352–8.
Kuin RA, et al. Diphenylcyclopropenone in patients with alopecia areata. A critically appraised topic. Br J Dermatol. 2015;173(4):896–909.
Sharma VK, Gupta S. Twice weekly 5 mg dexamethasone oral pulse in the treatment of extensive alopecia areata. J Dermatol. 1999;26(9):562–5.
Rashidi T, Mahd AA. Treatment of persistent alopecia areata with sulfasalazine. Int J Dermatol. 2008;47(8):850–2.
Alkhalifah A, et al. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62(2):191–202.
Aghaei S. An uncontrolled, open label study of sulfasalazine in severe alopecia areata. Indian J Dermatol Venereol Leprol. 2008;74(6):611–3.
Ellis CN, Brown MF, Voorhees JJ. Sulfasalazine for alopecia areata. J Am Acad Dermatol. 2002;46(4):541–4.
OShea, J.J., et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii111–5.
King B, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b–3 trial. Lancet. 2023;401(10387):1518–29.
Liu LY, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–8.
Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Investig Dermatol. 2014;134(12):2988–90.
Dhayalan A, King BA. Tofacitinib citrate for the treatment of nail dystrophy associated with alopecia universalis. JAMA Dermatol. 2016;152(4):492–3.
Mackay-Wiggan J, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15): e89790.
Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507.
European Medicines Agency. EMA confirms measures to minimise risk of serious side effects with Janus kinase inhibitors for chronic inflammatory disorders; 2023. https://www.ema.europa.eu/en/news/ema-confirms-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic [cited 20 February 2023].
Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(5):850–6.
Phan K, Ramachandran V, Sebaratnam DF. Methotrexate for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80(1):120-127.e2.
Lee D, et al. Treatment of severe alopecia areata: combination therapy using systemic cyclosporine A with low dose corticosteroids. Ann Dermatol. 2008;20(4):172–8.
Jabbari A, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Investig Dermatol. 2018;138(7):1539–45.
Wambier CG, Craiglow BG, King BA. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol. 2021;85(3):743–5.
Choi JW, et al. Simvastatin/ezetimibe therapy for recalcitrant alopecia areata: an open prospective study of 14 patients. Ann Dermatol. 2017;29(6):755–60.
Cervantes J, et al. Treatment of alopecia areata with simvastatin/ezetimibe. J Investig Dermatol Symp Proc. 2018;19(1):S25-s31.
U.S. Food and Drug Administration. IMURAN (azathioprine). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/016324s039lbl.pdf
U.S. Food and Drug Administration. Highlights of prescribing information - Cellcept (mycophenolate mofetil). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/050722s035,050723s035,050758s033,050759s041lbl.pdf
U.S. Food and Drug Administration. Azulfidine EN-tabs: sulfasalazine delayed release tablets, USP. Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/007073s125lbl.pdf
U.S. Food and Drug Administration. Highlights of prescribing information: XELJANZ® (tofacitinib). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf
U.S. Food and Drug Administration. HIGHLIGHTS OF PRESCRIBING INFORMATION: JAKAFI™ (Ruxolitinib). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
U.S. Food and Drug Administration. Highlights of prescribing information: Olumiant (baricitinib). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf
Pfizer. FDA and EMA Accept Regulatory Submission for Pfizer’s Ritlecitinib for Individuals 12 Years and Older with Alopecia Areata. 2022 [cited 2023 20 February]; Available from: https://www.pfizer.com/news/pressrelease/press-release-detail/fda-and-ema-accept-regulatory-submission-pfizers
Ramírez-Marín HA, Tosti A (2022) Evaluating the therapeutic potential of Ritlecitinib for the treatment of Alopecia Areata. Drug Des Devel Ther 16:363–374
U.S. Food and Drug Administration. VYTORIN ® (ezetimibe/simvastatin). Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021687s022lbl.pdf
U.S. Food and Drug Administration. Loniten® minoxidil tablets, USP. Drugs@FDA: FDA-Approved Drugs. Retrieved October 12, 2021, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/018154s026lbl.pdf
National Institutes of Health. Prednisone tablets USP. U.S. National Library of Medicine. Retrieved October 12, 2021, from https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=efbe4ea2-e9b2-4e4c-a1b3-a323f61905c0&type=display
Acknowledgements
Author Contributions
Mohammad Ibrahim Ahmad Fatani, Abdullah Alkhalifah, Amaal Farhan Salman Alruwaili, Aymen Hamed Salem Alharbi, Ru'aa Alharithy, Amr Mohammad Khardaly, Hajer Yousef Almudaiheem, Ahmed Al-Jedai and Maysa Tariq Yousef Eshmawi contributed to the concept, writing of the draft content related to their section, reviewed compiled drafts, and approved the final version for publication.
Funding
The Ministry of Health, Kingdom of Saudi Arabia funded this project. Work group received reasonable honoraria for activities related to this publication, and travel and accommodation expenses from the Ministry of Health, KSA. The journal’s Rapid Service Fee was also funded by the Ministry of Health, KSA.
Medical Writing and Editorial Assistance
Medical writing support was provided by Itkan Health Consulting (Sahar Shami, BSc, Fatema Dabdoub, MD) and was funded by The Ministry of Health, Kingdom of Saudi Arabia.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Conflict of Interest
All authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fatani, M.I.A., Alkhalifah, A., Alruwaili, A.F.S. et al. Diagnosis and Management of Alopecia Areata: A Saudi Expert Consensus Statement (2023). Dermatol Ther (Heidelb) 13, 2129–2151 (2023). https://doi.org/10.1007/s13555-023-00991-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00991-3