Abstract
Introduction
We sought to understand key symptoms of generalized pustular psoriasis (GPP) and to confirm the relevance to patients and content validity of the Psoriasis Symptom Scale (PSS) in GPP.
Methods
A targeted literature review and clinical expert interviews were conducted as background research. Patients were interviewed individually (involving concept elicitation and cognitive interviews), and a separate patient workshop was conducted to determine disease-specific symptoms of importance.
Results
Seven participants with moderate (n = 4), severe (n = 2), and mild (n = 1) GPP and clinician diagnosis were interviewed. During concept elicitation, all participants indicated that pustules may underlie other symptoms. Symptoms reported by all patients were pain, redness, itch, burning, and discomfort. The PSS symptoms of pain, itching, burning, and redness were reported by ≥ 86% of patients as most frequently experienced. Upon debriefing, the PSS was well understood. Relevance and importance of these symptoms was confirmed in the GPP patient workshop.
Conclusion
Participant feedback found the PSS measure to be relevant and easy to understand. The symptoms included in the instrument, pain, redness, itch, and burning, were most frequently reported, important, and well understood by patients. Study results provided support for the content validity of the PSS for use as endpoints in GPP clinical trials.
Plain Language Summary
Generalized pustular psoriasis (GPP) is a severe rare disease, including redness and boils that sometimes come with fever and other general symptoms. This study asked patients with GPP about their key symptoms, and whether the Psoriasis Symptom Scale (PSS) is relevant to them as patients. The PSS is a questionnaire with the symptoms pain, itching, burning, and redness. We searched the literature and interviewed clinical experts to guide the patient interviews. Patients were recruited through clinical sites and the National Psoriasis Foundation (NPF). The interviews discussed GPP symptoms and the PSS questionnaire. Patients with GPP were also asked about commonly experienced symptoms in a workshop. Most patients had moderate to severe GPP. Patients in both the interviews and workshop described experiencing pain, redness, itch, burning, and discomfort with their boils. During interviews, the patients said the PSS questionnaire was easy to understand. Patients in the workshop also found the PSS to be relevant and easy to understand. Patients agreed the symptoms in the PSS, pain, redness, itch, and burning, were common and important. Study results support the PSS for use with patients in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
No patient-reported outcome (PRO) instruments have been developed specifically for the rare condition of generalized pustular psoriasis (GPP), or other forms of pustular psoriasis and with the input of patients with GPP. |
The study sought to identify whether the Psoriasis Symptom Scale (PSS), which contains four common symptoms reported by patients, pain, burning, itch and redness, was suitable to use for patients with GPP. |
Most participants spontaneously described symptoms, such as pain, redness, itch, burning, and overlapping symptoms, or signs such as inflammation, discomfort, and irritation. |
Overall, participants provided positive feedback on the PSS and found the measure to be relevant, straightforward, and easy to understand, and most said they found no aspect of the measure to be confusing. |
The findings from this study provided strong support for the content validity of the PSS and the use of the measure in future GPP clinical trials. |
Introduction
Generalized pustular psoriasis (GPP) is a severe and rare disease [1], associated with erythema and pustules either with or without fever and other systemic symptoms [2, 3]. GPP may either be preceded by plaque psoriasis or arise de novo [4,5,6]. In the chronic course of GPP, pustules may persist or reappear all over the body. GPP flares often result in hospitalizations due to general malaise and comorbidity and can be life-threatening. The worldwide prevalence of GPP is poorly understood, but figures indicate that approximately two per million people in Europe are affected. The incidence of cases of GPP in Japan is estimated at approximately 0.6 per million people each year [7, 8].
Given the reported functional and symptomatic burden of GPP including pain, capturing the patients’ perspective is of particular importance in clinical studies in these diseases. However, as is frequently the case with rare diseases, there is a lack of adequate measures to do so in GPP. No patient-reported outcome (PRO) instruments have been developed specifically for GPP or other forms of pustular psoriasis. The only PRO questionnaire described in the literature developed for a rare psoriasis phenotype is the Palmar–Plantar Quality of Life Index (PPQLI) [9]. It was developed through chart review of patients with palmoplantar psoriasis, a review of multiple hand and foot questionnaires, and clinical expert feedback to capture how skin lesions of the hands and feet affect functioning and quality of life in these patients. The PPQLI, however, requires additional validation [10].
The recently developed Psoriasis Symptom Scale (PSS) contains four common symptoms reported by patients: pain, burning, itch, and redness. The content validity and psychometric properties of this PRO measure have been established in patients with plaque psoriasis [11, 12]. Given the lack of specific PROs for use with patients with GPP and the conceptual overlap of the four symptoms of the PSS with those described to be the clinical features of GPP, this study assessed whether the PSS may be a suitable instrument for use with patients with GPP. The primary objectives of the study were to: (1) understand the symptom experience of adults living with GPP, (2) assess the content validity and patient interpretation of the PSS, and (3) evaluate the appropriateness of the PSS for use as a PRO measure in clinical trials to generate patient experience data that can be used to support regulatory approval.
Methods
Study Design
This was a cross-sectional, qualitative study involving semistructured, cognitive interviews with adults with clinician-confirmed diagnosis of GPP living in the USA. The methods used in this study followed US Food and Drug Administration (FDA) and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) guidance for assessing the content validity of measures for use in new patient populations [10, 13]. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. An overview of the study stages is presented in Fig. 1.
Stage 1: Background Research: Literature Review and Clinician Interviews
A targeted literature review was conducted to examine literature published between 2007 and 2017 focusing on: (1) symptom PRO measures used in GPP clinical studies and (2) symptoms relevant to patients with GPP. The findings were validated in interviews with three clinical experts with notable clinical experience and research publications in GPP in separate, 1-h interviews. The information from the background research was used to inform the study protocol, data collection forms, and patient interview guide.
Stage 2: Patient Interviews
Study Participants
Institutional review board (IRB) approval of the study protocol was obtained through Chesapeake IRB (IRB: Advarra, PI: Rentz, protocol 00019943) prior to participant recruitment to comply with human participants research requirements. Participants were recruited from a dermatology clinic in Ohio, where study staff identified eligible participants from patient databases, charts, and/or daily appointment schedules. Additional participants were recruited from the National Psoriasis Foundation (NPF); respondents to an online survey who reported GPP and provided permission to be approached for future research were contacted and invited to participate in the study. All recruitment procedures complied with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations.
Study participants needed to be ≥ 18 years of age, have a confirmed medical diagnosis of GPP, not have a diagnosis of drug-induced GPP, acute generalized exanthematous pustulosis, or Stevens–Johnson syndrome, and not have a history of another chronic pain condition. All study participants provided written informed consent for their participation in the interview. The clinical information form was completed by the site clinicians and collected information on the participant’s GPP and plaque psoriasis history, treatment, and clinical characteristics. This information was used to describe the sample and assist with interpreting the results of the individual interviews.
Interviews
Participants completed one, hour-long interview in-person or by telephone. An experienced qualitative researcher conducted the interviews using a semistructured cognitive discussion guide that was designed to elicit symptom experiences from participants and assess the content validity of the PSS. The discussion guide included two sections: section 1 utilized concept elicitation, and section 2 used cognitive interview techniques. Section 1 included questions to understand the symptom experience of adults living with GPP. Section 2 included questions to evaluate the content validity and patient interpretation of the PSS, related specifically to: (1) the content coverage to ensure items cover symptoms associated with GPP, (2) the clarity of the items, and (3) how the participants interpreted the items. Participants completed the PSS before the interviewer asked the cognitive interview questions. All interviews were audio-recorded with participants’ permission.
At the end of the interview, participants completed a sociodemographic questionnaire, the Patient Benefit Index (PBI) [14]. Participants were remunerated US$100 after completion of the interview.
PRO Measures
The PSS measures patient-reported psoriasis symptoms [11] and consists of four items assessing severity of pain, itch, redness, and burning during the past 24 h. It uses a five-point severity scale as follows: 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe. The PSS items sum to an unweighted total score.
The PBI is a 23-item measure evaluating patient-relevant benefits in dermatological treatment [14]. The instrument asks about the importance of treatment goals at the present time and uses a five-point response scale. Response options are “not at all,” “somewhat,” “moderately,” “quite,” and “very.” This PRO measure was used to describe the sample and assist with interpreting the results of the individual interviews.
Stage 3: Patient Workshop
A 1-day workshop was conducted in June 2019 with patient advisors from GPP patient advocacy groups from Canada, Spain, South Korea, the Netherlands, and the USA. The aim of the workshop was to elicit patient input on a range of topics related to the conduct of trials in GPP, including their own symptom experience. Patients were also asked to rank the most burdensome symptoms they have experienced.
Analyses
Descriptive statistics (mean, standard deviation, frequency) were used to characterize the patient self-report data in terms of sociodemographic, self-reported disease, and responses to the PSS or PBI-S instruments. Descriptive statistics were also performed for the clinician-report of patient clinical characteristics.
A coding framework was developed based on the interview guide. The concept elicitation section of the transcripts (the symptoms or signs experienced) was coded. The cognitive interview section coded included study participant statements of their understanding or relevance of the PSS instructions, recall options, response options, and item concepts for each item of the PSS. A random sample of 20% of the coded transcripts were reviewed by a second team member to ensure consistency in coding.
A content analysis approach was used to analyze the interview data using ATLAS.ti version 8.1 qualitative analysis software. The coding and analysis organized the interview data by topic. Qualitative findings were summarized with exemplary quotes as well as frequencies and percentages as appropriate by participant or group.
Results
Stage 1: Background Research: Literature Review and Clinician Interviews
A total of 696 abstracts were identified from both the PubMed and Embase searches. Eleven articles were found during abstract screening which described symptom outcomes, symptom experience, or use of PROs or clinician-reported outcomes in GPP.
We identified a variety of symptoms occurring during the course of GPP due to recurrent, widespread erythematous patches, studded with pustules and accompanied by fever and pain in almost all patients [2]. Patients complained of joint pain, skin pain, and itch and/or an intense burning sensation that can cause extreme discomfort [15]. There were no symptom PROs identified specifically for patients with GPP.
Clinical experts reported that patients with GPP could experience redness and pustules covering their whole body that can be accompanied by systemic symptoms such as fever or fatigue. Symptom severity was described as potentially extreme and life-threatening, and quality of life could be diminished in this population.
Stage 2: Concept Elicitation and Cognitive Interviews
Sample Description
Seven interviews were conducted either in person or by telephone in Columbus, Ohio (n = 3, 43%), and by telephone with participants recruited through the NPF patient advocacy organization (n = 4, 57%). The median age of study participants was 58.6 years (range 40.0–70.0 years). Five were female (71%), none were Hispanic or Latino, and the majority were White (n = 4, 57%) or Black or African American (n = 3, 43%). Four participants rated their current overall health as good (n = 2, 29%) or very good (n = 2, 29%); three rated it as fair (43%). Six respondents reported their current GPP severity as moderate (n = 4, 57%) or severe (n = 2, 29%), and another respondent reported their current GPP as very mild (n = 1, 14%). Complete sociodemographic characteristics for the study sample are presented in Table 1.
Clinical and Disease Characteristics
All participants had a GPP diagnosis; one had a dual diagnosis of GPP and palmoplantar pustulosis based on clinician verification. Psoriatic arthritis and arthritis were the most common comorbidities. Clinician-reported clinical characteristics are presented in Table 2.
PBI
The PBI questionnaire results reported the areas in which study participants would most like to see treatment impact. Specifically, participants wanted treatment to resolve skin defects (100%), pain (86%), and itch (86%), and result in a normal everyday life (100%).
Concept Elicitation
Experience of GPP
All participants reported experiencing pain and redness (n = 7, 100%), followed by itching, burning, and discomfort (n = 6, 86%), and dryness/dry skin and soreness (n = 4, 57%). Several symptoms were spontaneously mentioned by most of the patients—pain, redness, itch, and burning. Probed symptoms were discomfort and irritation. Patients acknowledged that some symptoms were synonymous or overlapped with one another such as discomfort, pain, irritation, and soreness. Cracks, while an uncommon sign of GPP, were mentioned by one participant who said the underlying symptom of the cracks was pain. Symptom saturation was achieved by the third interview for all symptoms. Table 3 describes the symptom experiences of patients with GPP and includes illustrative quotes.
Impact of GPP
Participants described a variety of impacts to their daily life or quality of life due to GPP. Job duties, household chores, sleep, and mobility were most commonly affected by itching (n = 6, 86%), burning (n = 5, 71%), and pain (n = 4, 57%). Participants mentioned that itch affected sleep and that clothes were uncomfortable due to inflamed or sensitive skin from redness. Patients also reported that symptoms including pain, burning, and soreness on the feet affected walking. Other symptoms or feelings of embarrassment about their skin prevented participation in social activities, athletic activities involving the hands such as golf or baseball, or wearing heels, socks, or clothing.
Cognitive Debriefing of the PSS
PRO Results: PSS
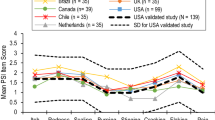
The mean PSS Total Score for the sample was 4.43 out of a total of 16 (Fig. 2a). For the overall sample, 70% of participants reported “none” or “mild” pain or redness symptoms, while 30% reported “moderate” or “severe” pain or redness in the 24 h preceding the interview. Fifty-seven percent of participants reported “none” or “mild” itch symptoms, while 43% reported “moderate” or “severe” itch in the past 24 h. The majority (n = 6, 86%) of participants reported “none” or “mild” burning symptoms; the remaining participant (n = 1, 14%) reported “severe” burning in the past 24 h. Full response details are listed in Table 4 and Fig. 2b.
Debriefing Results
Most participants (n = 5, 71%) indicated that the concepts were relevant and currently experienced symptoms.
-
100-003-GPP: “It was a good—I mean a good questionnaire, simple, good questions. I think all of them applied to me. I mean I might have put mild on those last three questions, but it’s, you know, it’s always there. So, yeah, it definitely applied to me."
-
100-010-GPP/PPP: “Definitely, yes… you’ve covered a lot of what people who have forms of psoriasis… have. They have pain, they’ve got redness, itching, burning, so no, I think they’re good."
Instructions
After completing the PSS, all participants (100%) commented that the instructions were clear. Several participants asked for clarification about whether they should think of their GPP or plaque psoriasis when answering the questions.
-
100-010-GPP: “Um, I guess you are asking me just about my psoriasis based on what other people have mentioned, and you want to know how my psoriasis compares to them. They have had 5 different levels, so you’re asking me what level I am in the last 24 h."
Response Scale
The response options for the four PSS symptom items—“none,” “mild,” “moderate,” “severe,” and “very severe”—were well understood by study participants and, overall, none had problems describing the difference between symptom severity using the scale.
-
002-022-GPP: “None-no pain. No pain, you feel like you ain’t in no pain…[Mild] means I’m on my medications, and it’s holding it at bay…[Moderate] means it would be a little bit rougher than mild. And severe…you can bear it, very severe is when I want to take … something for pain, you know?”
Recall Period
For each PSS question, participants were asked to specify what time period they considered when selecting an answer. Overall, participants focused on reporting for the last 24 h. Close to half of the participants indicated that the 24-h recall period made them think back to a specific time in the past 24 h when their symptoms were most severe.
-
100-010-GPP: “Pretty much from yesterday to today. Just pretty much I think about psoriasis all day long…I wonder if I can stand up and walk on my feet without them hurting, if I can take my shoes off without getting medicine all over the floor. I think about it all the time.”
Item-Level Feedback
Question 1: How severe was your pain due to your psoriasis during the past 24 h?
Severity of pain was generally understood by participants as painful skin, cracked skin, or pain during a flare-up. Participants clearly described their pain experience in the past 24 h, recounting experiences with “worst pain” or when their skin “flared up” to explain the intensity or severity of the pain.
-
100-010-GPP: “I selected mild because I’m at the point now where the pustules will pop and they’re purple and they’re going to start, they started to heal now, so I’d say it’s mild. It’s not the first thing I thought of when I woke up this morning, because I didn’t have pain this morning. You know, I knew I could just pop out of bed and not worry about what I’d put my feet on or anything. So, I said mild.”
This group explained their pain experience due to psoriasis in the past 24 h using words such as “irritating,” “discomfort,” and “sore.” They also mentioned how their levels of pain could vary.
Question 2: How severe was the redness from your psoriasis during the past 24 h?
Severity of redness was generally described by participants as raw skin, redness from inflammation, or skin peeling away. They described their redness in the past 24 h, as well as at its most severe. One participant, although not currently experiencing redness, stated that this symptom occurs due to inflammation. Another individual mentioned that redness is “a really bad irritation.”
-
002-001-GPP: “Well, I haven’t had any redness, so I selected none. But redness is definitely one of the things that you’ll experience when you’re broke [sic] out, because, again, it will be, you know, it’s red from being inflamed.”
Question 3: How severe was your itching from your psoriasis during the past 24 h?
Severity of itch was generally understood by participants as the need to scratch, dry skin itch, or itch that comes after skin is healing. Participants described the feeling of itch as well as the timing of itch during GPP flares. Some individuals associated itch with “when you’re broke out” or dry skin. Participants easily explained itch as “a deep tissue itch,” “itching like crazy,” “relentless,” and “you cannot get any relief.” They also identified itch specifically occurring on hands, feet, and legs. One participant stated that itch can occur in specific areas such as feet.
-
002-001-GPP: “I mean, like if I’m clearing up…you do itch…but at that point you can scratch because when you’re broke out you can’t scratch…so you’re able to get to the itch once you’re clearing up, so it’s not as bad, but when you’re broke out you can’t. There’s no way you, you’d just scratch the skin off, it’s like your skin has melted or something.”
Question 4: How severe was your burning from your psoriasis during the past 24 h?
Participants described their experience with burning due to psoriasis as when the “inside of your body is hot,” or “feeling like there is salt on your skin” or how a mosquito bite itches and burns. Another individual specifically stated his left foot was burning.
-
100-010-GPP/PPP: “Right now…this left foot is burning on the outer side of the foot, but…it doesn’t wake me up during the night and I don’t think about it during the day much now. This morning I haven’t thought about it much at all.”
These participants described burning as when “you can feel the heat” or being triggered by specific activities such as washing hair or putting hands in water. One individual described burning from psoriasis as something that is “active” and “not being controlled.” Other individuals mentioned burning resulting from scratching/itching as being inflamed or feeling like an infection.
Most Important Symptom to Improve with Treatment
Participants were asked which of their signs and symptoms would be most important to improve or reduce. Two participants identified itch (n = 2; 29%) as most important, and pustules, swelling, flaking, and losing heat were nominated once each as the most important symptom to improve or reduce. One participant mentioned it would be important to improve all their symptoms since everything was connected for them.
Stage 3: Patient Workshop
Eight GPP patient advisors and one carer of a GPP patient from the USA (n = 2 patients), Japan (n = 1 patient), Taiwan (n = 2 patients, n = 1 carer), and Malaysia (n = 3 patients) attended a face-to-face workshop. The objectives of the workshop were to learn about patient experiences and perspectives of the patient journey, including diagnosis, sign and symptom experience, burden and management of GPP. Only the section focusing on patients’ sign and symptom experiences is reported here.
When asked to rank the most burdensome signs, symptoms, and impacts, patients ranked itch first, followed by pustules and pain, being unable to sleep, fatigue and anxiety, joint pain and cracking skin, hot temper, plaques, fever, sticky skin, red skin, tight skin, and dry skin. Patients clarified that pustules ranked as the most burdensome sign of GPP, while itch and pain were considered to be symptoms of the pustules. Other burdensome signs or symptoms were redness and tightness, scaling, pumping and throbbing, dry skin, swelling, and depression.
Discussion
The overall aim of this study was to identify the most important symptoms of GPP in a sample with moderate to severe disease and explore whether the PSS may be an adequate measure to evaluate change in this disease. Most participants spontaneously described symptoms such as pain, redness, itch, burning, and overlapping symptoms or signs such as inflammation, discomfort, and irritation. Participants also reported experience with signs such as pustules, flaking, fissures, scaling, and peeling.
A workshop held with an international group of patients with GPP confirmed the findings of the qualitative study of US participants. Due to the small sample size, similarities or differences in GPP experience between adults from Asian countries compared with other countries could not be evaluated.
Overall, participants provided positive feedback on the PSS and found the measure to be relevant, straightforward, and easy to understand, and most said they found no aspect of the measure to be confusing. Participants had prior experience with the symptoms on the PSS, but most were not experiencing them at the time of the interview, saying they were not concurrently experiencing an episode or flare.
Participants were able to complete the questionnaire using the 24-h recall period. They understood and provided clear explanations for their selection of the response option for each PSS item and could provide clear distinctions between each response option for all four items. Most participants thought the scale worked well with the questions and would not modify the response options.
Overall, participants reported that the PSS was comprehensive and relevant to their experiences with GPP. The item meaning and response options were well understood for the items. As a result of cognitive interview feedback, no changes were made to the PSS instructions, recall period, response options, or items [11].
For rare disease populations, it is common to contend with small sample sizes. This study followed recommendations from the International Society of Pharmacoeconomics and Outcomes Research for conducting PRO validation in rare disease populations [16]. As such, efforts to enroll the small number of GPP study participants took 12 months utilizing diverse recruitment avenues (i.e. three clinical sites and the NPF) and included the evaluation of data from GPP workshop participants. The portion of the study sample recruited through the NPF represented a self-selected GPP population and constituted half of our study sample. As a result, some participants may not have had as many active symptoms as would be expected in the context of a treatment study. While almost all participants (86%) self-reported their disease as moderate or severe, most were not currently experiencing most of their symptoms.
Conclusions
Findings from this study support the content validity of the PSS and the use of the measure in future GPP clinical trials. This body of research provided evidence that the symptoms included in the PSS are important to and well understood by patients with moderate to severe GPP. Therefore, the measure is appropriate for inclusion in future studies designed to measure the effect of treatment for different forms of psoriasis.
References
DermNet NZ. Generalised pustular psoriasis 2014. https://dermnetnz.org/topics/generalised-pustular-psoriasis/. Accessed March 2020.
Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53(6):676–84.
Umezawa Y, Ozawa A, Kawasima T, Shimizu H, Terui T, Tagami H, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(Suppl 1):S43-54.
Zangrilli ATM, Saraceno R, Chimenti S. Clinical severity instruments. In: Chimenti S, editor. Psoriasis. Florence: Societa Editrice Italiana; 2005. p. 113–9.
Strober B, Kotowsky N, Medeiros R, Mackey RH, Harrold LR, Valdecantos WC, et al. Unmet medical needs in the treatment and management of generalized pustular psoriasis flares: evidence from a Survey of Corrona Registry Dermatologists. Dermatol Ther (Heidelb). 2021;11(2):529–41.
Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. 2013;27(8):1022–5.
Kharawala S, Golembesky AK, Bohn RL, Esser D. The clinical, humanistic, and economic burden of generalized pustular psoriasis: a structured review. Expert Rev Clin Immunol. 2020;16(3):239–52.
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21(3):355–70.
Farley E, Masrour S, McKey J, Menter A. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60(6):1024–31.
Food Drug Administration (FDA). Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Fed Regist. 2009;74(235):65132–3.
Rentz AM, Skalicky AM, Burslem K, Becker K, Kaschinski D, Esser D, et al. The content validity of the PSS in patients with plaque psoriasis. J Patient Rep Outcomes. 2017;1(1):4.
Rentz AM, Skalicky AM, Esser D, Zema C, Becker K, Bodhani A, et al. Reliability, validity, and the ability to detect change of the Psoriasis Symptom Scale (PSS) in patients with plaque psoriasis. J Dermatolog Treat. 2020;31(5):460–9.
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12(8):1075–83.
Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schafer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301(8):561–71.
National Psoriasis Foundation. About Psoriasis 2020. https://www.psoriasis.org/about-psoriasis. Accessed March 2020.
Benjamin K, Vernon MK, Patrick DL, Perfetto E, Nestler-Parr S, Burke L. Patient-reported outcome and observer-reported outcome assessment in rare disease clinical trials: an ISPOR COA Emerging Good Practices Task Force Report. Value Health. 2017;20(7):838–55.
Acknowledgements
This work was supported by funding from Boehringer Ingelheim, Ingelheim am Rhein, Germany. We thank Dr. Dennis Revicki for consulting on the PSS, Peter Chongpinitchai and Haylee Andrews for their assistance with data collection and Sandra Macker and Jun Chen for data analysis. Authors gratefully acknowledge the support of the NPF and clinicians in recruiting study participants and the patients who participated in the qualitative interview study, as well as patient advisors who participated in workshop meetings.
Funding
This work was supported by Boehringer Ingelheim, Ingelheim am Rhein, Germany. Boehringer Ingelheim will be funding the Rapid Service Fee.
Editorial Assistance
Editorial assistance for this work was provided by Amara Tiebout and Fritz Hamme, and was funded by Boehringer Ingelheim.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors participated in the study design. Anne Skalicky, Anne Rentz, Dirk Esser, Alan Menter, and Christian Thoma participated in data collection. Anne Skalicky and Anne Rentz performed the data analyses together with Dirk Esser, Tristan Gloede and Christian Thoma. All authors contributed to the writing of the manuscript. All authors have reviewed and approved the final manuscript.
Disclosures
The study sponsor was involved in the design of the study, interpretation of the data, and preparation, review, and approval of the manuscript. Anne Rentz and Anne Skalicky are employed by Evidera, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. As Evidera employees, they work with a variety of companies and organizations and are expressly prohibited from receiving any payment or honoraria directly from these organizations for services rendered. Dirk Esser, Tristan Gloede and Christian Thoma are employees of Boehringer Ingelheim. A. David Burden has received consulting fees from AbbVie, Almirall, Boehringer Ingelheim, Celgene, Janssen, LEO Pharma, Lilly, Novartis, and UCB; and payment or honoraria for lectures and presentations from Almirall, Boehringer Ingelheim, Janssen, and Lilly. Siew Eng Choon has received consulting fees from AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Sanofi, and UCB. Ulrich Mrowietz has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall, Amgen, Aristea, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Dr. Reddy’s, Eli Lilly, Foamix, Formycon, Immunic, Janssen, LEO Pharma, Medac, MetrioPharm, Novartis, Phi-Stone, Pierre Fabre, Sanofi-Aventis, UCB Pharma. Alan Menter reports being an advisory board member, consultant, investigator and speaker, and receiving grants and honoraria from Abbott Labs, Amgen, Janssen Biotech, Inc. and LEO Pharma; being an advisory board member and an investigator, and receiving grants and honoraria from Boehringer Ingelheim; being a consultant, investigator and speaker, and receiving grants and/or honoraria from Sun Pharma and UCB; being a consultant and investigator, and receiving honoraria from Novartis and Eli Lilly; and being an investigator and receiving grants from Celgene and Merck.
Compliance with Ethics Guidelines
Central institutional review board approval (IRB: Advarra, PI: Rentz, Pro00019943) was received for this study. The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability
The study data available upon request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Burden, A.D., Mrowietz, U., Skalicky, A.M. et al. Symptom Experience and Content Validity of the Psoriasis Symptom Scale (PSS) in Patients with Generalized Pustular Psoriasis (GPP). Dermatol Ther (Heidelb) 12, 1367–1381 (2022). https://doi.org/10.1007/s13555-022-00736-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00736-8