Abstract
Introduction
The burden of mild-to-moderate atopic dermatitis (AD) in the United Kingdom (UK) is not well understood. Long-lasting AD flares may lead to systemic inflammation resulting in reversible progression from mild to more severe AD. This study aimed to assess the clinical and economic burden of mild-to-moderate AD in the UK.
Methods
AD patients were identified in the Health Improvement Network (THIN) from 2013 to 2017 and propensity score matched to non-AD controls by demographics. Patients were identified based on continuous disease activity using validated algorithms and sufficient patient status to fully validate data integrity for the entire period. Mild-to-moderate AD patients were identified by using treatment as a surrogate. Demographics, clinical characteristics and healthcare resource use (HCRU) were obtained from THIN. Literature reviews were conducted to obtain additional outcomes. A cost-of-illness model was developed to extrapolate the burden in 2017 to the UK population and in subsequent years (2018–2022).
Results
In 2017, the prevalence of mild-to-moderate AD in THIN was 1.28%. These patients reported higher comorbidity rates and significantly higher (p < 0.0001) HCRU, encompassing mean general practitioner visits (5.57 versus 3.59), AD-related prescriptions (5.85 versus 0.68) and total referrals (0.97 versus 0.82) versus matched non-AD controls. The model projected total HCRU and drug excess costs of €462.99M over the 5 years. The excess cost decreased to €417.35M after excluding patients on very potent topical corticosteroids, who most likely had at least moderate disease. The excess costs increased to €1.21B and €7.06B when considering comorbidity burden and productivity losses, respectively.
Conclusion
Mild-to-moderate AD patients had higher comorbidity burden, HCRU and cost compared with matched non-AD controls. Overall, UK country-based economic burden was high given partly the high prevalence of this disease. Moreover, productivity burden and comorbidities had considerable impact on the economic burden, which further suggests the importance of optimal disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is currently limited information available from observational studies on the specific clinical and economic burden of mild-to-moderate atopic dermatitis (AD), which represents approximately 90% of all AD cases. |
This study aimed to assess the burden of mild-to-moderate AD in the UK. |
What was learned from the study? |
Mild-to-moderate AD patients had a higher comorbidity burden, healthcare resource utilisation (HCRU) and costs compared with matched non-AD controls in the UK primary care setting. |
A cost-of-illness model projected total HCRU and drug excess costs of €462.99M cumulatively for the 5-year time horizon at the UK population level. |
Productivity burden and comorbidities were found to have considerable impact on the economic burden of mild-to-moderate AD. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14261339.
Introduction
Atopic dermatitis (AD), or eczema, is a common inflammatory and chronic condition characterised by dry skin, erythema, lichenification and pruritus [1]. Its lifetime prevalence has increased between 1990 and 2010 in the UK [2] with current overall estimates ranging between 1.62% and 5% [3,4,5] for the overall population and between 5.9% and 14.2% [6] in children.
Primary care providers are commonly the first point of contact, and about 70% of mild-to-moderate AD patients can be effectively managed in this setting [7]. In the UK, the National Institute of Clinical Excellence guidelines for children and the National Eczema Society (NES) guidelines for all age groups recommend emollients as first-line therapies for AD management [8,9,10,11]. Short duration topical corticosteroids (TCS) are the recommended first-line treatment for AD flare-ups and the selection of TCS potency depends on disease location, age, disease severity and responsiveness [10, 12, 13]. A stepped approach matching potency of TCS with AD severity is recommended, indicating mild potency TCS for mild disease, moderate potency TCS for moderate disease and very potent TCS for a short term use in severe AD [10, 12, 13].
Studies have shown that AD is associated with a substantial comorbidity burden [14,15,16,17,18], predisposing patients to atopic comorbidities (an event that is referred to as ‘atopic march’) including asthma, allergic rhinitis and food allergies [19], and non-atopic comorbidities including anxiety, depression and cardiovascular disease [20]. The literature has also demonstrated that there is an association between allergic and neuropsychiatric comorbidities, with metabolic and lifestyle comorbidities (e.g. obesity) in patients with AD [21, 22].
Additionally, studies have shown that early AD treatment is essential in treating this skin disease, and may also delay or prevent the atopic march [23]. The literature has also demonstrated the negative impact of AD and the associated comorbidities on patients’ quality of life [24,25,26,27] and on work productivity [27, 28]. Additionally, AD patients have a higher economic burden compared with non-AD patients and, further, increasing disease severity is correlated with substantially higher healthcare resource utilisation (HCRU) and costs [27, 29].
Optimal use of basic skin care management is needed in patients at each severity level of AD to avoid inadequately controlled symptoms [30]. Studies have shown a considerable impact of AD on patients with poorly controlled disease [31]. The literature has demonstrated that, when healthy skin barrier integrity is compromised, environmental stressors including pathogens (e.g. Staphylococcus aureus) infiltrate this barrier activating the innate immune receptors [32]. This activation may trigger inflammation resulting in the onset of reversible AD flares. Frequent AD flares may affect the onset of systemic inflammation leading to progression from mild to clinically severe AD. Hence, it is important to quantify the clinical and economic burden of mild-to-moderate AD given its high prevalence.
There is currently limited information available from observational studies on the specific clinical and economic burden of mild-to-moderate AD in the UK, which represents approximately 90% of all AD cases [3]. To the best of the authors’ knowledge, no recent UK-based cost-of-illness studies have assessed the clinical and economic burden of mild-to-moderate AD patients compared with matched non-AD controls.
The primary objective of this study is to assess the clinical and economic burden of mild-to-moderate AD compared with matched non-AD controls in the UK. The secondary objective is to evaluate the impact of disease severity on this burden by considering a potentially ‘milder subgroup’. This study hypothesises that mild-to-moderate AD is associated with a substantial clinical and economic burden compared with matched non-AD control, which further increases when including the impact of productivity loss and the burden of comorbidities. This study further hypothesises a decreased burden when considering a potentially ‘milder subgroup’.
Methods
A stepwise methodology was applied to estimate the economic and clinical burden of mild-to-moderate AD, which been delineated in Fig. 1. Initially, a retrospective analysis of the Health Improvement Network (THIN) was conducted to estimate the demographic and clinical characteristics, and HCRU of propensity-score-matched mild-to-moderate AD and non-AD controls. Then, targeted literature searches were conducted to identify inputs which were not available in THIN (e.g. costs, productivity loss). Finally, a cost-of-illness model was developed to project the costs to the UK population and a subsequent 5-year time horizon.
Data and Ethics
THIN is an electronic medical records (EMR) database including anonymised general practitioner (GP) patient records in the UK [33]. THIN provides data on demographic and clinical characteristics, HCRU and drug acquisition, for a representative sample of around 5.7% of the UK population [34, 35]. The database collects primary care patient information from practices that use Vision; a general practitioner software package developed to facilitate and support practice management and patient care. The database is regularly updated and currently contains inputs from data collected in over 550 general practices [35].
Clinical data in THIN are catalogued using Read codes, a comprehensive and searchable classification scheme for medical conditions, symptoms, and important background information. THIN has been widely used for epidemiological research, and prior studies have validated algorithms that allowed identifying patients with AD in THIN [36]. This study was conducted using THIN version 1809 and analyses were performed in June 2019.
IQVIA Medical Research Data (IMRD), incorporating data from THIN, a Cegedim Database is a collection of de-identified patient records collected from primary care.Footnote 1 The data collection scheme is approved by the UK Research Ethics Committee (reference number: 18/LO0441). The protocol for this study was also reviewed and approved by an independent Scientific Review Committee ([SRC] Reference Number 19THIN033), and the study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Individual patient consent is not required for this type of study.
Study Design
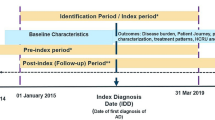
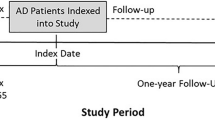
This retrospective analysis used a cross-sectional design to describe demographic and clinical characteristics of AD patients and to estimate HCRU by analysing data from the most recent complete years available (2013–2017) in THIN. Each individual calendar year, as well as the entire 5-year period, were evaluated to assess the cyclical nature of HCRU in AD. The study focused on the most recent individual calendar year (2017) to assess the nature of HCRU in AD.
Patients
AD patients in each time period were identified in THIN based on GP diagnosis of AD, continuous disease activity from 2013 to 2017 and sufficient patient status to fully validate data integrity for the entire time period (Table 1). Continuous disease activity was based on previously validated algorithms, which assessed the later of practice acceptable mortality recording, the information in Vision general practice system or patient registration date, and the earlier of patient transfer out date to practice last collection date [37].
As structured EMR lack information on disease severity, AD patients were stratified by disease severity using treatment as a surrogate severity measure. A published algorithm defined in a UK-based study by Silverwood et al. [38] was used to exclude severe patients and capture mild-to-moderate AD patients. Severe AD patients were excluded based on three criteria: systemic immunosuppressant treatment, a phototherapy code in the Clinical Practice Research Datalink (CPRD) or Hospital Episode Statistics; or AD-related referrals. Silverwood et al. considered patients to have mild AD by default and classified moderate AD based on a second potent TCS treatment within one year or a first topical calcineurin inhibitors (TCI) treatment.
Matched non-AD controls in each time period were identified in THIN based on the same criteria as AD patients except for the presence of AD diagnosis. To achieve this, a propensity-score-matching methodology was used. A logistic regression was performed on all patients (AD patients and matched non-AD controls, combined) to derive the propensity scores. The model included the variables age group, gender, and practice location; the propensity scores were the estimated probabilities that a patient belonged in the non-AD control group. The method of matching used was a greedy-match technique, with a calliper of 0.2 times the pooled standard deviation of the propensity scores, and with matching resulting in equal numbers in each group (PROC LOGISTIC and PROC PSMATCH in SAS version 9.4 were used).
In the UK, NES guidelines on AD treatment reserve very potent TCS for patients with severe AD[12], a criterion that was not captured in the Silverwood et al. algorithm. These guidelines state that AD patients who have received at least one TCS defined in the UK as ‘very potent’ (e.g. clobetasol propionate 0.05% and diflucortolone valerate 0.3%) would have severe disease [12]. Therefore, a fourth criterion that excluded patients who were treated with very potent TCS and who most likely had at least moderate AD as per the guidelines, was applied and explored in a secondary analysis.
Variables
Details of the variables included in this THIN analysis are available in Supplementary Tables S1–3. Demographic characteristics included age, sex, urban/rural classification, practice ID and Townsend code. Clinical characteristics included AD diagnosis and AD-related comorbidities. In order to assess the most relevant comorbidities in AD, common AD-related comorbidities were identified in the literature, validated through clinical expert insights, and aligned with corresponding frequencies in THIN during the entire study period. Subsequently, AD-related comorbidities in this study were confined by considering only comorbidities with a prevalence of 2.00% or higher (a threshold selected by consensus of all co-authors) in THIN.
HCRU was evaluated based on GP visits, total referrals, non-AD-related dermatology referrals, and AD-related prescriptions. GP visits included home, nurse, and telephone consultations. Non-AD related referrals were excluded using a specific THIN variable to align with the Silverwood et al. algorithm. Additionally, AD-related prescriptions were captured for emollients, TCI, TCS, topical antibiotics and topical antivirals. The drugs prescribed for AD patients were based on THIN drug codes using the British National Formulary (BNF) codes.
Statistical Analyses
Means, medians, and standard deviations were provided for continuous variables when performing descriptive analysis of continuous data, while numbers and percentages were provided for dichotomous and polychotomous variables when performing descriptive analysis of categorical data.
A generalised linear model was fitted for each of the HCRU variables (separate models), applying the normal distribution and the identity link. The independent variables included patient group (AD or control), practice location (England, Scotland, Northern Ireland and Wales), and their interaction; as well as age group (age 0–7, age 8–11, age 12–17, age 18+), and its interaction with patient group. Furthermore, gender and practice location were included as classification variables. Additionally, two summary measures of comorbidity were considered including ‘at least one metabolic-and-lifestyle’ comorbidity as a classification variable and Charlson Comorbidity Index (CCI) as a continuous variable. Mean differences were calculated (e.g. between AD patient and control group), along with 95% confidence intervals and associated p-values. It is noted here that the comorbidities had been aggregated and categorized into larger groups including (I) allergic comorbidities for AD, and (II) non-allergic comorbidities for AD. This latter was then divided into the following categories: (1) neuropsychiatric, (2) cardiovascular, (3) metabolic and lifestyle, (4) malignancies: lymphoma (adult), (5) skin infections, (6) eye disease and (7) autoimmune disease. All of these had been considered as classification variables but including them all into the models led to an ill-condition results (e.g. estimates were inflated). Through fitting multiple models and comparing results across dependent variables (in order to have a single set of independent variables for all of the dependent variables), ‘Metabolic and lifestyle’ alone remained in the models as a meaningful, statistically significant predictor. Based on clinical expert opinion and findings from the literature [21, 22], the metabolic and lifestyle category was considered as clinically relevant and predictors for the additional comorbidities included in the analysis (e.g. allergic and neuropsychiatric comorbidities).
Statistical analysis was developed and conducted in SAS.
Economics
The GP visit unit cost was identified from the Personal Social Services Research Unit [39] while referrals unit costs were derived from the National Health Services (NHS) Reference Costs from 2017 to 2018 [40]. Drug unit costs were sourced from the BNF 2018 [41] and the Monthly Index of Medical Specialties database 2018 [42].
Additional targeted literature reviews, using UK-specific sources, were conducted to identify productivity loss and comorbidity costs. The Ovid search platform was used to conduct the literature searches. The following databases were identified and used to conduct the searches: Excerpta Medica dataBASE (EMBASE), MEDLINE®, Cochrane Library, EconLit and NHS Economic Evaluation Database. The search strategies were defined in terms of the patient population, intervention and comparator, outcomes and study design (PICOS) framework. All literature searches were conducted on 25 June 2019. Details of the economic inputs, inclusion/exclusion criteria and the search terms are available in the Supplement and Tables S4–S10.
A cost-of-illness model was constructed to extrapolate the clinical and economic burden of mild-to-moderate AD and matched non-AD controls in THIN to the UK population in 2017 and subsequent years (2018–2022). Population estimates were obtained from the Office for National Statistics (ONS) [43, 44]. The primary analysis considered the burden of mild-to-moderate AD by excluding severe patients defined in the Silverwood et al. algorithm [38] and including HCRU and drug costs. The impact of disease severity was evaluated in a secondary analysis by excluding patients treated with very potent TCS who most likely had at least moderate AD as per the NES guidelines. Additionally, the burden of AD-related comorbidities and the impact of productivity loss due to AD were assessed in scenario analyses.
All costs have been inflated to 2018 and converted to euros (€) using the average ONS conversion rate (1.13) for 2018 [45].
Results
THIN Results for Unmatched AD and Non-AD Patients
The sample size and the baseline characteristics of the mild-to-moderate AD patients and non-AD patients prior to the propensity score matching in THIN are available in Table 2.
THIN Results for Matched AD Patients and Non-AD Controls
Baseline Characteristics
In the most recent calendar year, 2017, a total of 33,749 mild-to-moderate AD patients and 33,749 matched non-AD controls were identified in THIN (Table 3). Most patients were above 18 years old (55.09%) and based in England (42.79%). Given that the propensity score matching was based on patient demographics (including age, sex, socio-economic status and practice ID), baseline demographic characteristics were identical between AD patients and matched non-AD controls.
Clinical Results
In 2017, the prevalence of mild-to-moderate AD among the THIN population with continuous disease activity (N = 2,639,991) was 1.28% (Table 3). The prevalence was highest in childhood (5.11%) and decreased in adulthood (0.87%).
In 2017, mild-to-moderate AD patients had higher comorbidity rates compared with matched non-AD controls except for smoking (Table 4; Table S11 in the Supplementary Materials).
HCRU and Drug Acquisition Results
In 2017, mild-to-moderate AD patients reported statistically significantly higher HCRU including mean GP visits, AD-related prescriptions, total referrals and mean non-AD-related dermatology referrals compared with matched non-AD controls (Table 5). Emollients and TCS were the most commonly prescribed AD-related drugs among mild-to-moderate AD patients accounting for 62.13% and 34.89% of the total AD-related prescriptions, respectively.
Cost-of-Illness Model Results
The cost-of-illness model projected the economic burden of mild-to-moderate AD to the total UK population using ONS population estimates [43, 44] between 2018 and 2022. Overall, the model estimated a total of 859,014 mild-to-moderate AD patients in 2020 based on a constant yearly prevalence obtained in THIN (1.28%).
Primary Analysis
In 2020, mild-to-moderate AD patients incur substantially higher HCRU costs compared with matched non-AD controls encompassing GP visits (€202.37M versus €130.36M) and total referrals (€81.09M versus €68.51M). The costs per patient per year are shown in Fig. 2.
Per-patient HCRU cost for mild-to-moderate AD patients compared with matched non-AD control in 2020 (Primary analysis): This figure shows per-patient HCRU cost for mild-to-moderate AD patients compared with matched non-AD control (based on demographics including age, sex, socio-economic status and practice ID) in the primary analysis
In 2020, mild-to-moderate AD patients also incur substantial drug acquisition costs accounting for €8.04M, based on a per-patient cost of €9.36. Overall, the total HCRU and drug costs for mild-to-moderate AD patients compared with matched non-AD controls were €291.51M and €198.87M, respectively. The excess drug and HCRU cost at the UK population level was €462.99M over the total projected 5-year period (Table 6).
Secondary Analysis
In 2020, excluding AD patients treated with very potent TCS, who most likely had at least moderate AD, decreased the total costs of mild-to-moderate AD (n = 802,278) to €269.65M based on a per-patient cost of €336.11 (Table 6 and Fig. 3). The excess drug and HCRU costs decreased to €417.35M after excluding these patients compared with the primary analysis for the projected 5 years at the UK population level. The clinical and economic burden of comorbidities also decreased for these patients (Supplementary Tables S12–15 and Fig. S1).
Projected incremental costs for AD patients compared with matched non-AD control: excluding patients on very potent TCS who most likely had at least moderate AD (Secondary analysis): This figure depicts the projected incremental costs of AD patients compared with matched non-AD controls (based on demographics including age, sex, socio-economic status and practice ID) when excluding patients on very potent TCS who most likely had at least moderate AD as a secondary analysis
Scenario Analyses
In 2020, the burden of mild-to-moderate AD substantially increased when considering the financial impact of AD-related comorbidities (Tables 7, 8 and 9 and Figs. 4–5). Overall, the total costs of mild-to-moderate AD patients increased to €591.59M. The excess cost of mild-to-moderate AD patients compared with matched non-AD controls increased to €1.21B for the projected 5-years at the UK population level.
Projected incremental costs for mild-to-moderate AD patients versus matched non-AD controls, including comorbidity burden (scenario analysis): This figure shows the projected incremental costs for mild-to-moderate AD patients compared with matched non-AD controls (based on demographics including age, sex, socio-economic status and practice ID) when including the comorbidity burden in the scenario analysis
Incremental costs of all comorbidities for mild-to-moderate AD patients compared with matched non-AD controls for the projected time period (scenario analysis): This figure provides information on the incremental costs of all comorbidities of mild-to-moderate AD patients compared with matched non-AD controls (based on demographics including age, sex, socio-economic status and practice ID) for the projected time period in the scenario analysis
The impact of the productivity loss also had a substantial impact on the burden of mild-to-moderate AD (Table 9 and Fig. 6), increasing the associated total costs of mild-to-moderate AD to €8.06B aggregate for the projected 5 years at the UK population level.
Projected incremental costs of mild-to-moderate AD patients compared with matched non-AD control when considering the impact of the externally calculated productivity loss on the clinical and economic burden (the productivity loss was only applied for adults as no recent UK-based studies reported productivity loss for children and carers. Therefore, these estimates might be underestimated given lack of data for children and carers)* (scenario analysis): This figure depicts the projected incremental costs of mild-to-moderate AD patients compared with matched non-AD control (based on demographics including age, sex, socio-economic status and practice ID) when considering the impact of the externally calculated productivity loss on the clinical and economic burden in the scenario analysis
Discussion
This study suggests that mild-to-moderate AD, which represents approximately 90% of all AD cases [3], is associated with a substantial clinical and economic burden. Hence, this analysis addresses a previously identified research gap, where previous studies have been primarily focused to date on the burden of moderate-to-severe AD, although many AD patients are managed in primary care [46]. The findings in the present study provide a quantification of the burden of mild-to-moderate AD further demonstrating its considerable impact on the healthcare system in the UK.
The results of this retrospective THIN analysis showed that mild-to-moderate AD is associated with higher comorbidity rates and HCRU compared with matched non-AD controls at the primary care level in the UK. At a country-based level, the extrapolation of the observed incremental healthcare costs to the UK population using the cost-of-illness model, demonstrated a substantial burden of mild-to-moderate AD. The population-level burden substantially increased when the comorbidity burden and externally calculated productivity losses were also considered, which might further show the importance of optimal disease management of the disease. The study also suggests a decreased burden when considering a potentially ‘milder subgroup’. Therefore, this study is consistent with the tested hypotheses.
Generalisability
This study demonstrated that mild-to-moderate AD is associated with an increased public health burden given its high prevalence among the overall THIN population (1.28%). However, the overall prevalence estimated from this study was lower than previously reported estimates, ranging between 1.62% and 15% [3,4,5]. Compared with previous studies reporting AD prevalence, this study focused specifically on mild-to-moderate AD patients using EMR. Given the differences between the self-reported AD prevalence in the open population compared with physician-diagnosed disease in general practice, it is challenging to establish the true prevalence of mild-to-moderate AD [47]. Therefore, mild-to-moderate AD prevalence may be underestimated by only using physicians’ consultations. It can be suggested that clinicians should aim for improved identification and recording of AD and its severity in the primary care setting in order to ensure optimal management of the disease [32, 48].
The prevalence obtained from this analysis was the highest in childhood (5.11% among children between 0 and 7 years old) aligning with previously reported prevalence of children (5.9% and 14.2%)[6]. Given that parents and other family members are commonly involved in the care-giving of children with mild-to-moderate AD, the previous literature suggested that the burden of AD is shared by the patients and their families [49]. The detrimental impact of AD on the quality of life, the social, academic and occupational aspects on both patients and their families, as well as the burden on society due to higher costs and decreased productivity, have also been recognised in previous studies [46, 49]. Hence, the high prevalence of mild-to-moderate AD in children and the associated impact of the disease on the entire family unit, should be recognised when assessing the true burden of mild-to-moderate AD.
In this analysis, mild-to-moderate AD patients reported substantial HCRU and drug acquisition costs compared with matched non-AD controls. The main drivers of HCRU were GP visit costs, consistent with literature findings [50]. However, the AD-related dermatology referrals costs were not included in this evaluation to align with the algorithm by Silverwood et al., which may have underestimated the calculated HCRU. Hence, as mild-to-moderate AD is commonly treated in primary care, reducing GP visits through effective treatments might decrease primary care demand and also reduce the burden on the entire healthcare service [51].
The costs substantially increased when considering AD-related comorbidities, particularly asthma, bacterial skin infections, depression and sleep disorder; the impact of these comorbidities on the burden of AD has been recognised in previous studies [14, 52,53,54]. Despite its low frequency in THIN (1.38%), sleep disorder is estimated to account for substantial costs (€14,667,476.37 in 2020) at the UK population level. Additionally previous studies have shown that sleep disorders may result in increased levels of pruritus with associated poor school performance, family dysfunction and high Dermatology Life Quality Index scores [55,56,57,58], and may also worsen with disease severity[59]. Studies have also found that children with severe and persistent AD may face an increased risk of developing asthma and allergic rhinitis at a later life stage [60, 61]. Secondary skin infections caused by Staphylococcus aureus and Herpes simplex have also been associated with AD flares [62].
Based on the assumption that AD patients treated with very potent TCS had at least moderate disease, this analysis showed that more severe AD is possibly associated with a greater economic burden of comorbidities compared to milder AD. These findings are consistent with the conclusions from previous studies where increased disease severity was shown to be associated with a substantial increase in the frequency of comorbidities, which further associate with detrimental impact on quality of life and productivity loss [27, 63]. This notion would support the hypothesis that early diagnosis and treatment of mild-to-moderate AD may prevent the future development of associated comorbidities, which represent a significant burden to the health service [64].
Limitations
The main limitation of this study is related to the definition of severity used which required stratification of AD patients by disease severity using treatment as a surrogate measure for severity rather than using an objective clinical measure of severity. Data using established disease severity scoring measures, such as the Investigator Global Assessment (IGA), Eczema Area and Severity Index (EASI) or SCORing Atopic Dermatitis (SCORAD) are not routinely collected in the THIN database and therefore were not available for use in this study. Therefore, this study used a previously published algorithm for severity that was reviewed and supported by clinical expert opinion.
Based on further insights provided by clinical experts, this analysis assessed the impact of considering a potentially ‘milder subgroup’ on the burden by excluding patients treated with very potent TCS. Although this analysis did not allow for controlling for the impact of cofounding factors including site of application, patient age and previous treatment [12], the burden observed substantially decreased when excluding patients treated with very potent TCS. Therefore, it might be suggested that these patients have an increased clinical and economic burden, although the impact of cofounding factors on the burden remains uncertain.
Furthermore, this study focused on the most recent complete years available (2013–2017) in THIN at the time of the analysis and, hence, could not account for the use of dupilumab, which was recommended in the UK after the time period of our study [65].
As a targeted literature review did not identify any UK-specific sources reporting the incidence of mild-to-moderate AD, the AD population was estimated using the prevalence obtained from THIN. The economic burden of AD might be underestimated in this study given the previously reported incidence of 1.10% for mild-to-severe AD patients in England and Wales [5], which was not considered. When including this incidence in an exploratory analysis, it yielded a substantial increase in the economic burden of AD to €1.65B [5]. Therefore, it can be argued that the true clinical and economic burden of mild-to-moderate AD lies between €462.99M and €1.65B.
Additionally, THIN collates medical records collected at primary care, therefore this study could not capture secondary care data depicting the burden of moderate-to-severe AD. Previously, THIN data have been linked with Hospital Episode Statistics (HES) data, which provide the potential for linking primary and secondary care [35]. However, this was beyond the scope of the current study, which originally aimed to explore the burden of mild-to-moderate AD given its high prevalence. It can still be suggested that the estimates on the burden of mild-to-moderate AD are generalisable for the UK population as approximately 97% of AD patients are managed by their GP in the UK [7, 66], with THIN capturing primary care data for a representative sample of around 5.7% of the UK population [34].
Furthermore, while a comprehensive range of Read codes were applied to identify obesity in this analysis, the estimated frequency remains lower than expected. This underestimation is likely due to obesity being under-managed and recorded despite guidelines. This was concluded in a previous review which included studies that measured the proportion of adult patients with documented body mass index (BMI) or weight loss interventions in the UK across a range of regional and national databases [67]. This under-reporting is likely to underestimate the economic burden of AD-related comorbidities. Comorbidities such as asthma and ischaemic heart disease may be recorded more accurately given the nature of these conditions.
Finally, the costs of comorbidities, which were identified from the targeted literature search, entailed different cost components, and therefore the true burden of comorbidities remains uncertain. Given that mild-to-moderate AD patients had substantially higher comorbidity rates compared with matched non-AD controls, it can still be argued that the incremental burden of AD-related comorbidities remains substantial.
Conclusions
Mild-to-moderate AD patients had a higher comorbidity burden, HCRU and costs compared with matched non-AD controls. Excluding patients treated with very potent TCS, who most likely had at least moderate AD, decreased the clinical and economic burden of mild-to-moderate AD suggesting a possible link between disease burden and disease severity. Extrapolating the incremental healthcare costs of mild-to-moderate AD patients compared with matched non-AD controls to the UK population demonstrated a substantial country-based burden of mild-to-moderate AD, given in part the high prevalence of this disease. Moreover, productivity burden and comorbidities were found to have considerable impact on the economic burden. The increased burden observed in this study further suggests the importance of optimal disease management of mild-to-moderate AD.
Notes
IQVIA Medical Research Data (IMRD) incorporates data from THIN, A Cegedim Database. Reference made to THIN is intended to be descriptive of the data asset licensed by IQVIA”.
References
Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–22.
Deckers IA, McLean S, Linssen S, Mommers M, Van Schayck C, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PloS one. 2012;7(7):e39803.
Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson E, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–93.
Abuabara K, Ye M, McCulloch CE, Sullivan A, Margolis DJ, Strachan DP, et al. Clinical onset of atopic eczema: Results from 2 nationally representative British birth cohorts followed through midlife. J Allergy Clin Immunol. 2019;144(3):710–9.
Schofield J, Fleming D, Grindlay D, Williams H. Skin conditions are the commonest new reason people present to general practitioners in England and Wales. Br J Dermatol. 2011;165(5):1044–50.
Charman CRWH. Epidemiology. In: Bieber T, Leung DYM, editors. Atopic dermatitis. New York: Dekker; 2002. p. 21–42.
Eichenfield LF, Boguniewicz M, Simpson EL, Russell JJ, Block JK, Feldman SR, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136(3):554–65.
Cork MJ, Danby SG, Ogg GS. Atopic dermatitis epidemiology and unmet need in the United Kingdom. J. Dermatol. Treat. 2019;31(8):801–9.
National Eczema Society. Treating eczema – a stepped approach 2020. https://eczema.org/information-and-advice/treatments-for-eczema/. Accessed Apr 2020.
National Institute for Health and Care Excellence. Atopic eczema in under 12s: diagnosis and management 2007. https://www.nice.org.uk/guidance/cg57/chapter/1-Guidance. Accessed Apr 2020.
National Institute for Health Care and Excellence. Tacrolimus and pimecrolimus for atopic eczema. Technology appraisal guidance [TA82]. 2004. https://www.nice.org.uk/guidance/ta82/chapter/4-Evidence-and-interpretation. Accessed Apr 2020.
National Eczema Society. Topical corticosteroids factsheet. 2019. https://www.eczema.org/documents/578. Accessed Apr 2020.
Van Halewijn KF, Bohnen AM, Van Den Berg PJ, Pasmans SG, Bindels PJ, Elshout G. Different potencies of topical corticosteroids for a better treatment strategy in children with atopic dermatitis (the Rotterdam Eczema study): protocol for an observational cohort study with an embedded randomised open-label controlled trial. BMJ open. 2019;9(6):e027239.
Illi S, von Mutius E, Lau S, Nickel R, Gruber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925–31.
Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428–33.
Dalgard FJ, Gieler U, Tomas-Aragones L, Lien L, Poot F, Jemec GBE, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–91.
Schmitt J, Schwarz K, Baurecht H, Hotze M, Folster-Holst R, Rodriguez E, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137(1):130–6.
Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014:354250.
Dharma C, Lefebvre D, Tran M, Lou W, Subbarao P, Becker A, et al. Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy. 2018;48(1):48–59.
Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–51.
Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol. 2015;135(3):721-8.e6.
Ascott A, Mansfield K, Schonmann Y, Mulick A, Abuabara K, Roberts A, et al. Atopic eczema and obesity: a population‐based study. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.19597.
Huang A, Cho C, Leung DY, Brar K. Atopic dermatitis: early treatment in children. Curr TreatOpt Allergy. 2017;4(3):355–69.
Senra M, Wollenberg A. Psychodermatological aspects of atopic dermatitis. Br J Dermatol. 2014;170:38–43.
Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274-9.e3.
Sampogna F, Finlay AY, Salek SS, Chernyshov P, Dalgard F, Evers A, et al. Measuring the impact of dermatological conditions on family and caregivers: a review of dermatology-specific instruments. J Eur Acad Dermatol Venereol. 2017;31(9):1429–39.
Girolomoni G, Luger T, Nosbaum A, Gruben D, Romero W, Llamado LJ, et al. The economic and psychosocial comorbidity burden among adults with moderate-to-severe atopic dermatitis in europe: analysis of a cross-sectional survey. Dermatol Therapy. 2021;11(1):117–30.
Ariëns LF, Van Nimwegen KJ, Shams M, de Bruin DT, Van der Schaft J, Van Os-Medendorp H, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99(9–10):762–8.
Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the united states: analysis of healthcare claims data in the commercial, medicare, and medical databases. Adv Ther. 2017;34(8):1989–2006.
Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10-22.e2.
Zuberbier T, Orlow SJ, Paller AS, Taïeb A, Allen R, Hernanz-Hermosa JM, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226–32.
Domínguez-Hüttinger E, Christodoulides P, Miyauchi K, Irvine AD, Okada-Hatakeyama M, Kubo M, et al. Mathematical modeling of atopic dermatitis reveals “double-switch” mechanisms underlying 4 common disease phenotypes. J Allergy Clin Immunol. 2017;139(6):1861-72. e7.
Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–5.
Lo Re III, V, Haynes K, Forde KA, Localio AR, Schinnar R, Lewis JD. . Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharmacoepidemiol Drug Saf. 2009;18(9):807–14.
UCL. Identifying and appraising promising sources of UK clinical, health and social care data for use by NICE 2016. https://eppi.ioe.ac.uk/CMS/Portals/0/PDF%20reviews%20and%20summaries/Mapping%20real%20world%20data%202016%20Kneale%20report.pdf. Accessed Apr 2020.
Abuabara K, Magyari A, McCulloch CE, Linos E, Margolis DJ, Langan SM. Prevalence of atopic eczema among patients seen in primary care: data from the health improvement network. Ann Intern Med. 2019;170(5):354–6.
Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18(1):76–83.
Silverwood RJ, Forbes HJ, Abuabara K, Ascott A, Schmidt M, Schmidt SAJ, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ (Clinical research ed). 2018;361:k1786.
PSSRU. Unit costs of health and social care. Community-based health care staff. 2018. https://www.pssru.ac.uk/pub/uc/uc2018/community-based-health-care-staff.pdf. Accessed Oct 2019.
NHS. 2017/18 reference costs and guidance 2018. https://improvement.nhs.uk/resources/reference-costs/. Accessed Oct 2019.
British National Formulary. 2018. https://about.medicinescomplete.com/#/content/bnf/_351442497?hspl=Tacrolimus&hspl=undefined&hspl=ointment. Accessed Oct 2019.
Monthly Index of Medical Specialities. 2018. https://www.mims.co.uk/. Accessed Oct 2019.
Office for National Statistics. 2018. Principal projection - UK population in age groups. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/tablea21principalprojectionukpopulationinagegroups. Accessed Oct 2019.
Office for National Statistics. 2018 Population estimates. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Accessed Oct 2019.
Office for National Statistics. Average Sterling exchange rate: Euro 2021. https://www.ons.gov.uk/economy/nationalaccounts/balanceofpayments/timeseries/thap/mret. Accessed Feb 2021.
Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Investig Dermatol. 2017;137(1):26–30.
Pols DH, Wartna JB, Moed H, van Alphen E, Bohnen AM, Bindels PJ. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: a systematic review. Scand J Prim Health Care. 2016;34(2):143–50.
Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med. 2009;102(3):108–17.
Yang EJ, Beck KM, Sekhon S, Bhutani T, Koo J. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36(1):66–71.
Emerson R, Williams H, Allen B. What is the cost of atopic dermatitis in preschool children? Br J Dermatol. 2001;144(3):514–22.
Montgomery HE, Haines A, Marlow N, Pearson G, Mythen MG, Grocott M, et al. The future of UK healthcare: problems and potential solutions to a system in crisis. Ann Oncol. 2017;28(8):1751–5.
Lansang P, Lara-Corrales I, Bergman JN, Hong CH, Joseph M, Kim VHD, et al. Approach to the assessment and management of pediatric patients with atopic dermatitis: a consensus document. Section IV: consensus statements on the assessment and management of pediatric atopic dermatitis. J Cutan Med Surgery. 2019;23(5_suppl):32s-s39.
Alexander H, Paller A, Traidl-Hoffmann C, Beck L, De Benedetto A, Dhar S, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol. 2020;182(6):1331–42.
Schonmann Y, Mansfield KE, Hayes JF, Abuabara K, Roberts A, Smeeth L, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8(1):248-57.e16.
Blume-Peytavi U, Metz M. Atopic dermatitis in children: management of pruritus. J Eur Acad Dermatol Venereol. 2012;26:2–8.
Chrostowska-Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2013;27(2):e239–42.
Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol. 2007;87(4):295–8.
Schmitt J, Chen CM, Apfelbacher C, Romanos M, Lehmann I, Herbarth O, et al. Infant eczema, infant sleeping problems, and mental health at 10 years of age: the prospective birth cohort study LISAplus. Allergy. 2011;66(3):404–11.
Jeon C, Yan D, Nakamura M, Sekhon S, Bhutani T, Berger T, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Therapy. 2017;7(3):349–64.
Saunes M, Øien T, Dotterud CK, Romundstad PR, Storrø O, Holmen TL, et al. Early eczema and the risk of childhood asthma: a prospective, population-based study. BMC Pediatr. 2012;12(1):168.
von Kobyletzki LB, Bornehag C-G, Hasselgren M, Larsson M, Lindström CB, Svensson Å. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. 2012;12(1):11.
Ong PY, Leung DY. The infectious aspects of atopic dermatitis. Immunol Allergy Clin. 2010;30(3):309–21.
Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–48.
Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Phys. 2012;86(1):35–42.
National Institute for Health and Care Excellence. Treating eczema in people over 12 [updated 2 March 2021]. https://pathways.nice.org.uk/pathways/eczema#path=view%3A/pathways/eczema/treating-eczema-in-people-over-12.xml&content=view-index. Accessed Mar 2021.
Abuabara K, Magyari AM, Hoffstad O, Jabbar-Lopez ZK, Smeeth L, Williams HC, et al. Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Investig Dermatol. 2017;137(8):1655–62.
McLaughlin JC, Hamilton K, Kipping R. Epidemiology of adult overweight recording and management by UK GPs: a systematic review. Br J Gen Pract. 2017;67(663):e676–83.
Acknowledgements
Clinical validation was provided by Suvi Hokkanen, MD, UK. Expertise on the THIN database was provided by Mustafa Dungarwalla, UK.
Funding
This study and article processing charges were sponsored by Pfizer Inc.
Authorship
The authors are responsible for all content and editorial decisions, had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Medical writing assistance
Editorial and medical writing support under the supervision of the co-authors was provided by Mary Greenacre, an independent medical writer and communications consultant, and was funded by IQVIA, in accordance with Good Publication Practice (GPP3) guidelines.
Prior presentation
The results of this study have been previously submitted in an abstract, which was presented in an e-poster at the EADV Virtual Congress in October 2020.
Disclosures
Miss Toron, currently employed by Bristol-Myers Squibb, reports personal fees from Pfizer Inc., during the conduct of the study; Dr. Neary, currently employed by Pfizer Inc., reports personal fees from Pfizer Inc., during the conduct of the study; personal fees from Pfizer Inc., outside the submitted work; Timothy Smith, currently employed by Novartis Pharmaceuticals Corporation, reports personal fees from Pfizer Inc., during the conduct of the study; personal fees from Pfizer Inc., outside the submitted work; Dr. Gruben, currently employed by Pfizer Inc., reports personal fees from Pfizer Inc., during the conduct of the study; personal fees from Pfizer Inc., outside the submitted work; Dr. Romero, currently employed by Pfizer Ltd., reports personal fees from Pfizer Ltd., during the conduct of the study; personal fees from Pfizer Ltd., outside the submitted work; Dr. Cha, currently employed by Pfizer Inc., reports personal fees from Pfizer Inc., during the conduct of the study; personal fees from Pfizer Inc., outside the submitted work;.Mr. Patel, currently employed by Daiichi Sankyo UK Ltd., reports personal fees from Pfizer Inc., during the conduct of the study; Miss Vasileva, currently employed by IQVIA, reports personal fees from Pfizer Inc., during the conduct of the study; Dr. Ameen, currently employed by Royal Free London National Health Services Foundation Trust UK,reports other from Pfizer Ltd, other from Pfizer Inc, outside the submitted work.
Compliance with Ethics Guidelines
IQVIA Medical Research Data (IMRD), incorporating data from THIN, a Cegedim Database is a collection of de-identified patient records collected from primary care (“IQVIA Medical Research Data (IMRD) incorporates data from THIN, A Cegedim Database. Reference made to THIN is intended to be descriptive of the data asset licensed by IQVIA”). The data collection scheme is approved by the UK Research Ethics Committee (reference number: 18/LO0441). The protocol for this study was also reviewed and approved by an independent Scientific Review Committee (SRC Reference Number 19THIN033), and the study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Individual patient consent is not required for this type of study.
Data availability
The datasets generated during and/or analysed during the study are not publicly available due to a restriction in access through strict data sharing agreements, which require an approved protocol through SRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Farah Toron, Timothy W. Smith, Keyur Patel: at the time of the analysis.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Toron, F., Neary, M.P., Smith, T.W. et al. Clinical and Economic Burden of Mild-to-Moderate Atopic Dermatitis in the UK: A Propensity-Score-Matched Case–Control Study. Dermatol Ther (Heidelb) 11, 907–928 (2021). https://doi.org/10.1007/s13555-021-00519-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00519-7