Abstract
Introduction
The objective of this study was to conduct a retrospective analysis to understand the patient profile, treatment patterns, healthcare resource utilization, and cost of atopic dermatitis (AD) of patients eligible for targeted therapy in Taiwan.
Methods
A retrospective, claims-based analysis was undertaken using Taiwan’s National Health Insurance Research Database from 01 January 2014 to 31 December 2017. Patients aged ≥ 2 years and with at least one diagnosis code for AD during 2015 were identified. Patients with comorbid autoimmune diseases were excluded. Enrolled AD patients were categorized using claims-based treatment algorithms by disease severity and their eligibility for targeted therapy treatment. A cohort of targeted therapy-eligible patients was formed, and a matched cohort using patients not eligible for targeted therapy was derived using propensity score matching based on age, gender, and the Charlson Comorbidity Index (CCI). Treatment patterns, resource utilization, and costs were measured during a 1-year follow-up period.
Results
A total of 377,423 patients with AD were identified for this study. Most patients had mild AD (84.5%; n = 318,830) with 11.9% (n = 45,035) having moderate AD, and 3.6% (n = 13,558) having severe AD. Within the 58,593 moderate-to-severe AD patients, 1.5% (n = 897) were included in the targeted therapy-eligible cohort. The matched cohort consisted of 3558 patients. During the 1-year follow-up period, targeted therapy-eligible patients utilized antihistamines (85.5%), topical treatments (80.8%), and systemic anti-inflammatories (91.6%) including systemic corticosteroids (51.4%) and azathioprine (59.1%). During the first year of follow-up, targeted therapy-eligible patients (70.5%; 7.01 [SD = 8.84] visits) had higher resource utilization rates and frequency of AD-related outpatient visits compared with the matched cohort (40.80%; 1.85 [SD = 4.71] visits). Average all-cause direct costs during 1-year follow-up were $2850 (SD = 3629) and $1841 (SD = 6434) for the eligible targeted therapy and matched cohorts, respectively. AD-related costs were 17.7% ($506) of total costs for the targeted therapy eligible cohort and 2.2% ($41) for the matched cohort.
Conclusions
AD patients eligible for targeted therapy in Taiwan experienced high resource and economic burden compared with their non-targeted-therapy-eligible counterparts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The objective of this study was to conduct a retrospective analysis to understand the patient profile, treatment patterns, healthcare resource utilization, and cost of AD patients eligible for targeted therapy in Taiwan. |
Taiwan’s National Health Insurance Research Database (NHIRD), a population-based claims database covering > 99% of Taiwan’s population was used in this analysis. The NHIRD records all entries for claims for reimbursement of medical services and materials. |
Targeted therapy-eligible AD patients in Taiwan experienced high resource and economic burden compared with their non-biologic-eligible counterparts. |
Though < 1% of patients with AD are eligible for targeted therapy in Taiwan, an unmet need exists for these patients because they have significant resource utilization and cost burden compared with those not eligible for targeted therapy. |
Introduction
Atopic Dermatitis (AD) is a chronic inflammatory skin disease characterized by frequent flares of eczematous lesions associated with severe itching [1]. Although the exact pathophysiology remains unclear, it is understood that there is a misdirected immune reaction, and interactions between environmental factors and genetic predisposition enter into the determinism of AD [2, 3].
The global prevalence of AD has been estimated to range between 2% and 20%, with significant age and regional variations [4].
AD has the highest disease burden among skin diseases, as measured by disability-adjusted life-years (DALY) [5]. The global DALY rate for patients with AD was reported at 121 in 1990, and remained similar in 2017 at 123 [5]. Studies have reported an elevated risk of many comorbidities including major depression, any depressive disorder, and anxiety disorders [6,7,8]. A cross-sectional study in the USA demonstrated that patients with AD reported higher proportions of having only fair/poor overall health (25.8% versus 15.8%), being somewhat/very dissatisfied with life (16.7% versus 11.4%), and lower mental and physical health scores compared with those without AD [9]. In addition, a claims database analysis in the USA reported patients with AD had higher healthcare resource utilization, including emergency room (ER) visits, outpatient visits, and pharmacy prescriptions, and increased mean total per patient costs than non-AD controls [10].
Though there is currently no cure for AD, the American Academy of Dermatology (AAD) recommend topical corticosteroids (TCs) as the mainstay of antiinflammatory therapy [11]. Topical calcineurin inhibitors (TCI) are recommended as a second-line therapy for short-term and noncontinuous chronic treatment of non-immunocompromised AD patients who have failed to respond to other topical prescription, or when they are not recommended [11]. The European guidelines for the treatment of AD recommend TCs and TCI for both flare management and long-term proactive therapy [12]. The European guidelines also suggest the use of systemic immunosuppressants in severe refractory cases, and biologicals as an option [12].
The Taiwanese Dermatological Association recommends emollients, TCs, antihistamines, and therapeutic patient education as first-line treatment options for AD in their 2020 consensus statement [13]. Second-line options include TCI, burst use of systemic corticosteroids, phototherapy, and topical and systemic antibiotics. Lastly, third-line treatments are systemic immunomodulatory agents, antiseptics, and alternative medicine.
The objective of this study was to describe the patient profile, treatment utilization, healthcare resource utilization, and direct costs of AD patients eligible for targeted therapy in Taiwan.
Methods
Data Source
Taiwan’s National Health Insurance Research Database (NHIRD), a population-based claims database covering > 99% of Taiwan’s population was used in this analysis. The NHIRD records all entries for claims for reimbursement of medical services and materials. As an insurance claims database, it does not include clinical information such as laboratory test results, physical examination findings, or diagnostic testing. However, basic demographic information such as the age and gender of patients can be determined.
The database includes reimbursement data for outpatient, inpatient, ambulatory, and pharmacy claims that are accompanied by International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) codes and the amount (New Taiwan dollar, NT$) of the claims. Additionally, the dates of the inpatient visit, outpatient visit, or hospital admission/discharge are recorded. Pharmacy orders include drug names, strength, dose, quantity, and date of dispensing. The study was granted an exemption from ethical review by the Taipei Medical University-Joint Institutional Review Board.
Study Design
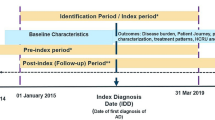
This was a retrospective study utilizing data from 1 January 2014 to 31 December 2017, which was the last year of available data at the time of institutional review board submission. Note that in addition to the typical 2-year lag in data availability from the NHIRD, data access was delayed for almost 2 years due to COVID-19 restrictions closing the data centers. There was an index period running from 1 January 2015 to 31 December 2016 to enroll AD patient in 2015 and to categorize AD patients by disease severity 1 year after the enrollment date in 2015. A pre-index period spanning 365 days prior to the enrollment date was also included to examine the presence of any exclusion diagnoses and assess patients’ baseline characteristics for the analysis. Patients enrolled in the study had a minimum of 1 year of follow-up available (see Fig. 1).
AD patients enrolled in this study were classified as having either mild, moderate, or severe disease during the mandatory 1 year follow-up period. Patients with moderate or severe disease were evaluated individually to assess their eligibility for targeted therapy. Patients were placed into the targeted therapy eligible cohort based on criteria recommended by local clinical experts. A cohort of mild, moderate, and severe patients was matched to the targeted therapy-eligible cohort.
Patients of all severities not eligible for targeted therapies and the matched cohort were indexed on the date of their first AD diagnosis during the index period. The index date for eligible targeted therapy patients coming from the severe AD cohorts was the initial date of an included systemic immunosuppressant (azathioprine, cyclosporine, methotrexate, or mycophenolate). The index date for eligible targeted therapy patients from the moderate AD cohort was the initial date of an included systemic immunosuppressant or phototherapy during the index period, whichever was initiated first.
Population
Patients with AD during the index period were first identified and enrolled in the study if they fit the following inclusion and exclusion criteria:
Inclusion Criteria
-
≥ 1 Primary or secondary healthcare claims for atopic dermatitis (ICD-9-CM codes: 691.8) in 2015.
-
Patients with age ≥ 2 years old.
Exclusion Criteria
-
Less than 365 days of enrollment in the NHIRD before or after index date
-
Patients with any systemic immunosuppressant treatment for other comorbid autoimmune diseases in the 1 year before the first date of AD diagnosis in 2015 (enrollment date), including inflammatory bowel disease (along with ulcerative colitis [ICD-9-CM codes: 556 or 564.1] and Crohn’s disease [ICD-9-CM codes: 555]), lupus erythematosus (ICD-9-CM codes: 710), rheumatoid arthritis (ICD-9-CM codes: 714), psoriatic arthritis (ICD-9-CM codes: 696.0 or 696.1), psoriasis (ICD-9-CM codes: 696.1), ankylosing spondylitis (ICD-9-CM codes: 720.x), and non-infectious uveitis (ICD-9-CM codes: 364.04), or having undergone organ transplantation (ICD-9-CM codes: V42).
AD patients enrolled into the study were divided into one of three subgroups based on disease severity, using an algorithm previously developed by Cho et al. [8]. Severe AD patients included AD patients meeting one of the following criteria in the 1-year period post-index date (1) ≥ 3 claims for systemic immunosuppressants or systemic corticosteroids, or (2) ≥ 3 claims for superpotent TCs (i.e., clobetasol) combined with ≥ 3 claims for phototherapy. Non-severe AD patients were classified as moderate if they met any of these criteria during the 1-year follow-up period: (1) ≥ 1 claim for systemic corticosteroids, (2) ≥ 3 claims for superpotent TCs, or (3) ≥ 1 claim for phototherapy. Patients who neither met the criteria for severe, nor those for moderate AD were labeled as mild patients. Note that mild patients eligible for targeted therapies in the follow-up period were excluded.
Furthermore, a cohort of moderate-to-severe patients that would be eligible for targeted therapy for AD was developed. Moderate patients were included as eligible for targeted therapy if they had used any systemic immunosuppressant for AD for 1–89 days and/or ≥ 6 claims for phototherapy during the 12 months after enrollment date. Severe patients were deemed eligible targeted therapy patients if they received a systemic immunosuppressant for AD for ≥ 90 days during the 1-year follow-up period after the enrollment date.
Lastly, a cohort of AD patients who were not eligible for targeted therapy was developed and included patients with mild, moderate, or severe AD that did not meet the criteria for targeted therapy at any point during the follow-up period. These patients were then matched with AD patients who were eligible for targeted therapy to compare the disease burden in a 1 (eligible targeted therapy) to 4 (non-eligible targeted therapy) ratio using propensity score methods based on age, gender, and Charlson Comorbidity Index (CCI) scores.
Measurement
Demographics were described at the index date, and clinical characteristics (comorbidities) were described during the 365-day pre-index period for all included cohorts. Demographics included age and gender, and clinical characteristics included comorbidities within the Charlson Comorbidity Index (CCI) [14] and other comorbidities commonly associated with AD [10]. Patients were considered to have a comorbidity if they had ≥ 1 inpatient or ≥ 3 ambulatory claim(s) associated with the comorbidity’s diagnostic code(s) during the pre-index period. The diagnosis codes for the individual components of the CCI and the other comorbidities are included in the Supplementary Material (S1. Table 1).
Treatment utilization during the 1-year follow-up period was also measured for the patients who were eligible for targeted therapy and the matched cohort. Classes of medications and therapies included antihistamines, topical treatments, systemic antiinflammatory therapies, systemic antibiotics, phototherapy, and traditional Chinese medicine. Within topical treatments, corticosteroids, calcineurin inhibitors, and antibiotics were examined individually. Systemic antiinflammatory therapies included corticosteroids, azathioprine, cyclosporine, methotrexate, and mycophenolate. Patients were classified as having been on a medication if they had one or more pharmacy claims during the follow-up period. The codes for these medications and therapies can be found in the Supplementary Material (S1. Table 2).
Healthcare resource utilization and direct medical costs were also measured during the mandatory 1-year follow-up period for targeted therapy-eligible and the matched cohorts. Healthcare resource utilization included only AD-related resources and was measured for hospital admission, hospital days, outpatient visits, and emergency room visits. Healthcare resource utilization was measured as the percentage of patients with one or more claims, and the average number of visits/days were reported for patients with at least one claim during follow-up. Direct costs, which were all costs reimbursed by the National Health Insurance Administration, were measured as both all-cause and AD-related. AD-related costs were broken down into medication (pharmacy) and non-medication costs, as well as inpatient, outpatient, and emergency room costs. All costs were converted from TWD to USD using an exchange rate of 1 TWD = 0.036 USD.
Statistical Analysis
. Descriptive analyses were conducted to describe patient profile, treatment utilization, healthcare resource utilization, and direct costs. In the analyses of continuous variables, descriptive statistics were presented for the number of observations, mean and standard deviation (SD). In the analyses of categorical variables, descriptive statistics including frequencies and percentages were tabulated. Testing for statically significant differences without multiplicity adjustments was done between the eligible targeted therapy and matched cohorts using Student t-tests for continuous variables and Chi-square tests for categorical variables. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
There were a total of 379,745 Taiwanese aged ≥ 2 years with AD claims for reimbursement in the database in 2015 (Fig. 2). Of these patients 377,423 (99.4%) were enrolled in the study as they did not meet any of the exclusion criteria. Enrolled AD patients were primarily categorized as suffering from mild AD (n = 318,830; 84.5%), followed by moderate (n = 45,035; 11.9%), and severe AD (n = 13,558; 3.6%). Patients aged 2–18 years accounted for 34.1% of all AD patients included in the study. The percentage of patients aged 2–18 years was lower within severe AD patients (27.9%) compared with mild (34.1%) and moderate (32.9%).
The eligible targeted therapy cohort included 897 patients, in which there were 629 patients with severe AD and 268 with moderate AD The matched cohort consisted of 3588 patients non eligible for targeted therapy.
Patient demographics and clinical characteristics (comorbidities) stratified by AD severity are presented in Table 1. The mean age of patients increased from 33.6 (SD = 23.7) years in mild patients to 35.8 (SD = 24.5) years in moderate, and 38.6 (SD = 24.7) years in severe patients. Patients were mostly female in the cohort of all AD patients (54%), mild patients (55%), and moderate patients (51%). Demographics and clinical characteristics for the eligible targeted therapy and matched cohorts are presented in Table 2. The eligible targeted therapy cohort had an average age of 34.8 (SD = 19.0) years and were mostly male (61%), which was in-line with the matched cohort (age: 34.9 (SD = 19.2) years; male: 61.4%).
Of the AD-related atopic comorbidities measured (asthma, allergic rhinitis, allergic conjunctivitis, and urticaria), all had a higher prevalence in eligible targeted therapy patients compared with the matched cohort. Eligible targeted therapy patients also had higher prevalence of ocular disorder, and viral, fungal, and bacterial infection than the matched cohort.
Treatment Utilization
Treatment utilization by class during the 1-year follow-up period was mostly greater in moderate and severe AD patients than in mild AD patients (Table 3). Antihistamines were utilized by 21.9% of patients with mild AD compared with 88.7% and 97.2% of moderate and severe patients, respectively. Topical corticosteroid utilization was highest (74.4%) in patients with moderate AD, followed by patients with severe AD (65.7%) and mild AD (16.6%). In line with the patient severity definitions, the largest difference in utilization rates across the severities was observed in systemic antiinflammatory corticosteroids, which were utilized by 96.5% of severe AD patients, 64.6% of moderate AD patients, and 1.1% of mild AD patients. Lastly, traditional Chinese medicine utilization was similar across severities, with severe patients having the highest utilization rate (5.4%) followed by mild (4.6%) and moderate patients (4.3%). Supplementary Table 3 (S1. Table 3) presents the treatment utilization stratified by patients aged 2–18 years, and those over 18 years. As in the non-stratified analysis, medication usage increases by severity across most treatments, and the most common treatments were antihistamines and topical treatments. There were some differences across the age cohorts: for most patients across all severities, treatment utilization was greater for those aged 2–18 years versus those over 18 years old; for moderate and severe patients, most patients using traditional Chinese medicines were aged 2–18 years, with the utilization rate higher than those above 18 years. Corticosteroids are preferred in the oral form versus the intravenous (IV) form for both those aged 2–18 years and those over 18 years old.
During the 1-year follow-up period, eligible targeted therapy patients had significantly higher utilization rates of most treatments, including antihistamines (85.5% versus 46.4%; p < 0.0001), topical treatments (80.8% versus 36.6%; p < 0.0001), systemic antiinflammatory medications (91.6% versus 19.1%; p < 0.0001), and phototherapy (17.1% versus 0.6%; p < 0.0001) compared with the matched cohort because of the definition of the cohorts during patient selection (Table 4).
Of patients that used topical treatments in the eligible targeted therapy cohort, 74.8% utilized corticosteroids and 22.4% utilized calcineurin inhibitors, whereas in the matched cohort, 35.2% utilized corticosteroids and less than 10% utilized calcineurin inhibitors. The most frequently utilized systemic antiinflammatory medications were azathioprine (59.1%), corticosteroids (51.4%), and methotrexate (43.1%) among the targeted therapy eligible cohort.
Healthcare Resource Utilization
AD-related healthcare resource utilization was higher for the eligible targeted therapy than the matched cohort during the 1-year follow-up period (Fig. 3). A greater percentage of eligible targeted therapy patients had one or more claims for an AD-related hospital admission (3.3% versus 0.2%; p < 0.0001), emergency room visit (1.5% vs. 0.2%; p < 0.0001), or outpatient visit (70.5% vs. 40.8%; p < 0.0001) compared with the matched cohort.
Among the users of the measured resources, average AD-related frequency of use in hospital admission, ER visits, and outpatient visits was numerically higher for the eligible targeted therapy patients compared with the matched cohort (Table 5). The average number of hospital admissions was 2.59 (SD = 2.92) for eligible targeted therapy patients compared with 2.14 (SD = 1.07) in the matched cohort. Outpatient visits were markedly higher for the eligible targeted therapy cohort, with 9.97 (SD = 9.03) compared with 4.53 (SD = 6.53) for the matched cohort.
Direct Costs
The average all-cause direct costs during the the 1-year follow-up period were $2850 (SD = 3629) and $1841 (SD = 6434) for the eligible targeted therapy and matched cohorts, respectively. AD-related costs were $506 (17.7% of total all-cause costs) for the targeted therapy eligible cohort and $41 (2.2%) for the matched cohort (Fig. 4).
For both the eligible targeted therapy and matched cohorts, AD-related outpatient costs dominated total AD-related costs, with 93.6% ($473) and 94.5% ($38) of AD-related costs, respectively (Fig. 4). The distribution of costs between AD-related inpatient and emergency room was similar between the cohorts.
When total AD-related costs were divided by medication compared with non-medication, there was a striking difference in the distribution between the cohorts. Targeted therapy eligible patients averaged 56.8% of AD-related costs in AD-related medication compared with only 32.6% for the matched cohort.
Discussion
This study evaluated the demographics, comorbidities, treatment utilization, healthcare resource utilization, and costs of AD patients in Taiwan eligible for targeted therapy over a 1-year period. To the authors’ knowledge, this is the first study to identify AD patients eligible for targeted therapy in Taiwan and to compare their treatment utilization and resource use/costs over time with non-targeted therapy eligible AD patients.
The prevalence of moderate and severe AD patients was 11.9% and 3.6%, respectively. The percentage of moderate-to-severe patients eligible for targeted therapy was estimated to be 0.24% of all AD patients based on the criteria recommended by local clinical experts.
There were subtle differences among the demographic variables in this analysis, another recent analysis using the NHIRD in Taiwan [8], and an analysis of AD patients in Japan [15]. The mean age of AD patients was younger in the analysis by Cho et al. (25.11 versus 34 years in this analysis and 34.15 years in the Japanese analysis). However, the percentage of all AD patients being male was 44.5% in this analysis, 46.8% in Cho et al. [8], and 55.0% in the Japanese analysis. There were also differences in asthma rates (4.89% versus 1.99%) and allergic rhinitis rates (10.8% versus 1.72%) in our study versus that of Cho et al. [8]
The distribution of all AD patients by disease severity was similar in this analysis compared with the Cho et al. analysis, which used the same algorithm. There was a slightly higher percentage of patients with mild AD (84.5% versus 73.3%) in our analysis, which led to smaller groups of moderate (11.9% versus 19.3%) and severe patients (3.6% versus 7.4%). These differences could potentially be explained by the inclusion criteria of AD definition. In our study we included patients who had one claim with AD diagnosis in 2015, whereas in the previous study by Cho et al., patients were included if they had at least two AD diagnoses or one AD diagnosis with one diagnosis of generalized dermatitis in 2010. These differences could also be explained by the use of the full database in our analysis compared with the one million patient sample in the Cho et al. study. There were many consistencies observed between these two studies, such as an increase in the proportion of adults for moderate and severe cases compared with mild cases, and a greater percentage of male patients in the moderate and severe cohorts compared with the mild cohort.
However, this small number of patients represents a significant burden to the healthcare system compared with their non-eligible counterparts. Targeted therapy-eligible patients were more likely to use each class of medication than the matched cohort. Targeted therapy-eligible patients also utilized resources at greater rates and frequency than the matched cohort, and had AD-related direct costs that were 12.5 times higher. This elevated level of resource utilization suggests that targeted therapy-eligible patients had a higher disease burden and are in need of effective therapies.
Our analysis included sensitivity analysis around subgroups of different age groups: less than 18 years old versus 18 years and older. The higher use of both topical and systemic antibiotics in children and adolescents suggest a higher infection rate in that age group. Traditional Chinese medicine is driven by the under 18 years age group in moderate-to-severe patients, which might be because parents choose to avoid adverse events associated with Western medicine. The previous study by Lee et al., using Taiwan’s NHIRD and the same criteria defining disease severity, demonstrated that AD patients with higher disease severity incurred higher outpatient, medication, and total costs [16]. While our study did not include designations for controlled versus uncontrolled AD, the results were consistent with the literature in that the targeted therapy-eligible patients, which may be similar to uncontrolled patients, had a higher economic burden than the matched cohort.
As this analysis is the first of its kind to examine the incremental burden of targeted therapy-eligible AD patients compared with non-targeted therapy-eligible AD patients, the results have important implications for stakeholders. While there were no targeted therapies for AD available in Taiwan during the timeframe of this study, this analysis shows a large incremental resource and cost burden for targeted therapy-eligible patients. The reimbursement and implementation of effective medications to treat these patients is desperately needed as the prevalence of AD increases in Asia [17].
As with all retrospective claims-based analyses, there are several limitations to this study. First, the study is subject to miscoding in the dataset and thus misdiagnosis. Furthermore, without clinical information, the reliance on ICD codes can also lead to misdiagnosis. Second, the algorithms for disease severity and targeted therapy eligibility are based on claims rather than clinical outcomes and may not be representative of commonly utilized disease activity and severity metrics used in clinical practice. Additionally, the algorithm developed to identify patients eligible for targeted therapy has not been previously used or validated in the literature. Lastly, the target therapy eligibility algorithm to evaluate the eligibility of patients during 2015 and 2016, when no targeted therapies were available. The reimbursement guidelines had not yet been published during this timeframe. The publication of these guidelines could have changed the care patterns of moderate-to-severe AD patients, and this analysis may not be representative of the current number of patients and treatment patterns, and future studies may reflect more accurately current regulations and practices.
Conclusions
Less than 1% of patients with AD eligible for targeted therapy in Taiwan were considered in this analysis. However, an unmet medical need exists in the treatment paradigm for these patients, and they carry a significant resource utilization and cost burden compared with their counterparts not eligible for targeted therapy.
References
Heratizadeh A, Werfel T. Anti-inflammatory therapies in atopic dermatitis. Allergy. 2016;71(12):1666–75.
Roesner LM, Werfel T, Heratizadeh A. The adaptive immune system in atopic dermatitis and implications on therapy. Expert Rev Clin Immunol. 2016;12(7):787–96.
Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45(3):566–74.
Mohn CH, Blix HS, Halvorsen JA, Nafstad P, Valberg M, Lagerløv P. Incidence trends of atopic dermatitis in infancy and early childhood in a nationwide prescription registry study in Norway. JAMA Netw Open. 2018;1(7): e184145.
Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–9.
Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. American Academy of Dermatology Guidelines: awareness of comorbidities associated with atopic dermatitis in adults. J Am Acad Dermatol. 2022;86(6):1335-1336.e18.
Cheng CM, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, et al. Risk of developing major depressive disorder and anxiety disorders among adolescents and adults with atopic dermatitis: a nationwide longitudinal study. J Affect Disord. 2015;178:60–5.
Cho Y-T, Hsieh W-T, Chan TC, Tang C-H, Chu C-Y. Prevalence of baseline comorbidities in patients with atopic dermatitis: a population-based cohort study in Taiwan. JAAD Int. 2020;1(1):50–8.
Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7.
Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, medicare, and Medi-Cal Databases. Adv Ther. 2017;34(8):1989–2006.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Wollenberg A, Kinberger M, Arents B, Aszondi N, Avila Valle G, Barbarot S, et al. EuroGuiDerm guideline on atopic eczema, Version 1.0, June 2022.
Chan TC, Wu NL, Wong LS, Cho YT, Yang CY, Yu Y, et al. Taiwanese Dermatological Association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120(1 Pt 2):429–42.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Igarashi A, Fujita H, Arima K, Inoue T, Dorey J, Fukushima A, et al. Health-care resource use and current treatment of adult atopic dermatitis patients in Japan: a retrospective claims database analysis. J Dermatol. 2019;46(8):652–61.
Lee EM, Cho YT, Hsieh WT, Chan TC, Shen D, Chu CY, et al. Healthcare utilization and costs of atopic dermatitis in Taiwan. J Formos Med Assoc. 2022. https://doi.org/10.1016/j.jfma.2022.01.028.
Tsai TF, Rajagopalan M, Chu CY, Encarnacion L, Gerber RA, Santos-Estrella P, et al. Burden of atopic dermatitis in Asia. J Dermatol. 2019;46(10):825–34.
Acknowledgements
Funding
This study (including journal fees) was funded by Eli Lilly & Co.
Author Contributions
CHT, CYC, CYW, KJN, YHH, TT, PYC, and BW conceived and designed the analysis and contributed to the writing of the manuscript.
Disclosures
PYC and BW are paid consultants to Eli Lilly and Co. CYW, KJN, YHH, and TT are employees of Eli Lilly and Co. CYC is an investigator, consultant, speaker, and/or advisory board member for AbbVie, Dermira, Eli Lilly and Co., Janssen, Mylan, Novartis, Oneness Biotech, Novartis, Pfizer, Regeneron, Roche, Sanofi, United BioPharma, and Viatris. CHT has nothing to disclose.
Compliance with Ethics Guidelines
The study was granted an exemption from ethical review by the Taipei Medical University-Joint Institutional Review Board.
Data Availability
Data sharing is not applicable to this article as no new datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tang, CH., Huang, YH., Chuang, PY. et al. Patient Characteristics, Treatment Patterns, Healthcare Resource Utilization, and Costs of Targeted Therapy-Eligible Atopic Dermatitis Patients in Taiwan—A Real-World Study. Dermatol Ther (Heidelb) 12, 2547–2562 (2022). https://doi.org/10.1007/s13555-022-00816-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00816-9