Abstract
Introduction
The goal of this study was to investigate how concurrent shear stress and tensile strain affect endothelial cell biomechanical responses.
Methods
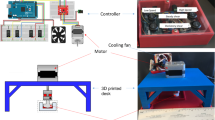
Human coronary artery endothelial cells were exposed to concurrent pulsatile shear stress and cyclic tensile strain in a programmable shearing and stretching device. Three shear stress–tensile strain conditions were used: (1) pulsatile shear stress at 1 Pa and cyclic tensile strain at 7%, simulating normal stress/strain conditions in a healthy coronary artery; (2) shear stress at 3.7 Pa and tensile strain at 3%, simulating pathological stress/strain conditions near a stenosis; (3) shear stress at 0.7 Pa and tensile strain at 5%, simulating pathological stress/strain conditions in a recirculation zone. Cell morphology was quantified using immunofluorescence microscopy. Cell surface PECAM-1 phosphorylation, ICAM-1 expression, ERK1/2 and NF-κB activation were measured using ELISA or Western blot.
Results
Simultaneous stimulation from pulsatile shear stress and cyclic tensile strain induced a significant increase in cell area, compared to that induced by shear stress or tensile strain alone. The combined stimulation caused significant increases in PECAM-1 phosphorylation. The combined stimulation also significantly enhanced EC surface ICAM-1 expression (compared to that under shear stress alone) and transcriptional factor NF-κB activation (compared to that under control conditions).
Conclusion
Pulsatile shear stress and cyclic tensile strain could induce increased but not synergistic effect on endothelial cell morphology or activation. The combined mechanical stimulation can be relayed from cell membrane to nucleus. Therefore, to better understand how mechanical conditions affect endothelial cell mechanotransduction and cardiovascular disease development, both shear stress and tensile strain need to be considered.








Similar content being viewed by others
References
Amaya, R., L. M. Cancel, and J. M. Tarbell. Interaction between the stress phase angle (SPA) and the oscillatory shear index (OSI) affects endothelial cell gene expression. PLoS ONE 11(11):e0166569, 2016.
Amaya, R., A. Pierides, and J. M. Tarbell. The interaction between fluid wall shear stress and solid circumferential strain affects endothelial gene expression. PLoS ONE 10(7):e0129952, 2015.
Azuma, N., S. A. Duzgun, M. Ikeda, H. Kito, N. Akasaka, T. Sasajima, and B. E. Sumpio. Endothelial cell response to different mechanical forces. J. Vasc. Surg. 32(4):789–794, 2000.
Azuma, N., S. A. Duzgun, M. Ikeda, H. Kito, N. Akasaka, T. Sasajima, and B. E. Sumpio. Endothelial cell response to different mechanical forces. J. Vasc. Surg. 32(4):789–794, 2000.
Birukova, A. A., S. Chatchavalvanich, A. Rios, K. Kawkitinarong, J. G. Garcia, and K. G. Birukov. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am. J. Pathol. 168(5):1749–1761, 2006.
Breen, L. T., P. E. McHugh, and B. P. Murphy. Multi-axial mechanical stimulation of HUVECs demonstrates that combined loading is not equivalent to the superposition of individual wall shear stress and tensile hoop stress components. J. Biomech. Eng. 131(8):081001, 2009.
Breen, L. T., P. E. McHugh, and B. P. Murphy. HUVEC ICAM-1 and VCAM-1 synthesis in response to potentially athero-prone and athero-protective mechanical and nicotine chemical stimuli. Ann. Biomed. Eng. 38(5):1880–1892, 2010.
Chatterjee, S., E. A. Browning, N. Hong, K. DeBolt, E. M. Sorokina, W. Liu, M. J. Birnbaum, and A. B. Fisher. Membrane depolarization is the trigger for PI3 K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 302(1):H105–114, 2012.
Chatterjee, S., K. Fujiwara, N. G. Perez, M. Ushio-Fukai, and A. B. Fisher. Mechanosignaling in the vasculature: emerging concepts in sensing, transduction and physiological responses. Am. J. Physiol. Heart Circ. Physiol. 308(12):H1451–1462, 2015.
Cheng, J. J., B. S. Wung, Y. J. Chao, and D. L. Wang. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension 28(3):386–391, 1996.
Chiu, Y.-J. Identification of the kinase for flow-and stretch-elicited phosphorylation of platelet endothelial cell adhesion molecule-1 in endothelial cells. Thesis (Ph.D.), University of Rochester. School of Medicine and Dentistry. Dept. of Biochemistry and Biophysics, 2008. http://hdl.handle.net/1802/6626.
Conway, D. E., M. T. Breckenridge, E. Hinde, E. Gratton, C. S. Chen, and M. A. Schwartz. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23(11):1024–1030, 2013.
Correa-Meyer, E., L. Pesce, C. Guerrero, and J. I. Sznajder. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 282(5):L883–891, 2002.
Dancu, M. B., and J. M. Tarbell. Large Negative Stress Phase Angle (SPA) attenuates nitric oxide production in bovine aortic endothelial cells. J. Biomech. Eng. 128(3):329–334, 2006.
Dewey, Jr., C. F., S. R. Bussolari, M. A. Gimbrone, Jr., and P. F. Davies. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103(3):177–185, 1981.
Doriot, P. A., P. A. Dorsaz, L. Dorsaz, E. De Benedetti, P. Chatelain, and P. Delafontaine. In-vivo measurements of wall shear stress in human coronary arteries. Coron. Artery Dis. 11(6):495–502, 2000.
Dorland, Y. L., and S. Huveneers. Cell–cell junctional mechanotransduction in endothelial remodeling. Cell. Mol. Life Sci. 74(2):279–292, 2017.
Fujioka, K., N. Azuma, H. Kito, V. Gahtan, K. Esato, and B. E. Sumpio. Role of caveolin in hemodynamic force-mediated endothelial changes. J. Surg. Res. 92(1):7–10, 2000.
Fujiwara, K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J. Intern. Med. 259(4):373–380, 2006.
Gambillara, V., C. Chambaz, G. Montorzi, S. Roy, N. Stergiopulos, and P. Silacci. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am. J. Physiol. Heart Circ. Physiol. 290(6):H2320–2328, 2006.
Gawlak, G., S. Son, Y. Tian, J. J. O’Donnell, 3rd, K. G. Birukov, and A. A. Birukova. Chronic high-magnitude cyclic stretch stimulates EC inflammatory response via VEGF receptor 2-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 310(11):L1062–1070, 2016.
Golledge, J., R. J. Turner, S. L. Harley, D. R. Springall, and J. T. Powell. Circumferential deformation and shear stress induce differential responses in saphenous vein endothelium exposed to arterial flow. J. Clin. Invest. 99(11):2719, 1997.
Gulino-Debrac, D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1(2):e24180, 2013.
Hajra, L., A. I. Evans, M. Chen, S. J. Hyduk, T. Collins, and M. I. Cybulsky. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. 97(16):9052–9057, 2000.
Hashimoto, K., N. Kataoka, E. Nakamura, K. Tsujioka, and F. Kajiya. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis. 194(2):e9–e17, 2007.
Hay, D. C., C. Beers, V. Cameron, L. Thomson, F. W. Flitney, and R. T. Hay. Activation of NF-kappaB nuclear transcription factor by flow in human endothelial cells. Biochim. Biophys. Acta 1642(1–2):33–44, 2003.
Heart Disease Facts American Heart Association. Heart Disease and Stroke Update 2015. Cited 2 February 2016. http://www.theheartfoundation.org/heart-disease-facts/heart-disease-statistics/.
Heuslein, J. L., J. K. Meisner, X. Li, J. Song, H. Vincentelli, R. J. Leiphart, E. G. Ames, B. R. Blackman, B. R. Blackman, and R. J. Price. Mechanisms of amplified arteriogenesis in collateral artery segments exposed to reversed flow direction. Arterioscler. Thromb. Vasc. Biol. 35(11):2354–2365, 2015.
Hsiai, T. K., S. K. Cho, H. M. Honda, S. Hama, M. Navab, L. L. Demer, and C. M. Ho. Endothelial cell dynamics under pulsating flows: significance of high versus low shear stress slew rates (d(tau)/dt). Ann. Biomed. Eng. 30(5):646–656, 2002.
Hsu, H. J., C. F. Lee, and R. Kaunas. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS ONE 4(3):e4853, 2009.
Hunt, B. J., and K. M. Jurd. Endothelial cell activation. A central pathophysiological process. BMJ. 316(7141):1328–1329, 1998.
Hurley, N. E., L. A. Schildmeyer, K. A. Bosworth, Y. Sakurai, S. G. Eskin, L. H. Hurley, and L. V. McIntire. Modulating the functional contributions of c-Myc to the human endothelial cell cyclic strain response. J. Vasc. Res. 47(1):80–90, 2010.
Hwang, J., M. H. Ing, A. Salazar, B. Lassegue, K. Griendling, M. Navab, A. Sevanian, and T. K. Hsiai. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ. Res. 93(12):1225–1232, 2003.
Jalali, S., Y.-S. Li, M. Sotoudeh, S. Yuan, S. Li, S. Chien, and J. Y. Shyy. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18(2):227–234, 1998.
Jo, H., K. Sipos, Y. M. Go, R. Law, J. Rong, and J. M. McDonald. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J. Biol. Chem. 272(2):1395–1401, 1997.
Johnson, B. D., K. J. Mather, and J. P. Wallace. Mechanotransduction of shear in the endothelium: basic studies and clinical implications. Vasc. Med. 16(5):365–377, 2011.
Juan, S. H., J. J. Chen, C. H. Chen, H. Lin, C. F. Cheng, J. C. Liu, M. H. Hsieh, Y. L. Chen, H. H. Chao, T. H. Chen, P. Chan, and T. H. Cheng. 17beta-estradiol inhibits cyclic strain-induced endothelin-1 gene expression within vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 287(3):H1254–1261, 2004.
Keegan, P. M., S. Anbazhakan, B. Kang, B. S. Pace, and M. O. Platt. Biomechanical and biochemical regulation of cathepsin K expression in endothelial cells converge at AP-1 and NF-kappaB. Biol. Chem. 397(5):459–468, 2016.
Kevil, C. G., A. W. Orr, W. Langston, K. Mickett, J. Murphy-Ullrich, R. P. Patel, D. F. Kucik, and D. C. Bullard. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J. Biol. Chem. 279(18):19230–19238, 2004.
Kohn, J. C., D. W. Zhou, F. Bordeleau, A. L. Zhou, B. N. Mason, M. J. Mitchell, M. R. King, and C. A. Reinhart-King. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys. J . 108(3):471–478, 2015.
Kou, B., J. Zhang, and D. R. Singer. Effects of cyclic strain on endothelial cell apoptosis and tubulogenesis are dependent on ROS production via NAD(P)H subunit p22phox. Microvasc. Res. 77(2):125–133, 2009.
Kumar, A., E. W. Thompson, A. Lefieux, D. S. Molony, E. L. Davis, N. Chand, S. Fournier, H. S. Lee, J. Suh, K. Sato, Y.-A. Ko, D. Molloy, K. Chandran, H. Hosseini, S. Gupta, A. Milkas, B. Gogas, H.-J. Chang, J. K. Min, W. F. Fearon, A. Veneziani, D. P. Giddens, S. B. King, B. De Bruyne, and H. Samady. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J. Am. Coll. Cardiol. 72(16):1926–1935, 2018.
Kyriakis, J. M., and J. Avruch. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92(2):689–737, 2012.
Levesque, M. J., and R. M. Nerem. The elongation and orientation of cultured endothelial cells in response to shear stress. J. Biomech. Eng. 107(4):341–347, 1985.
Li, Y.-S. J., J. H. Haga, and S. Chien. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 38(10):1949–1971, 2005.
Li, L. F., B. Ouyang, G. Choukroun, R. Matyal, M. Mascarenhas, B. Jafari, J. V. Bonventre, T. Force, and D. A. Quinn. Stretch-induced IL-8 depends on c-Jun NH2-terminal and nuclear factor-kappaB-inducing kinases. Am. J. Physiol. Lung Cell. Mol. Physiol. 285(2):L464–475, 2003.
Li, Y.-S., J. Shyy, S. Li, J. Lee, B. Su, M. Karin, and S. Chien. The Ras-JNK pathway is involved in shear-induced gene expression. Mol. Cell. Biol. 16(11):5947–5954, 1996.
Liu, Z., J. L. Tan, D. M. Cohen, M. T. Yang, N. J. Sniadecki, S. A. Ruiz, C. M. Nelson, and C. S. Chen. Mechanical tugging force regulates the size of cell-cell junctions. Proc. Natl Acad. Sci. U.S.A. 107(22):9944–9949, 2010.
Lu, D., and G. S. Kassab. Role of shear stress and stretch in vascular mechanobiology. J. R. Soc. Interface 8(63):1379–1385, 2011.
Maniotis, A. J., C. S. Chen, and D. E. Ingber. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl Acad. Sci. U.S.A. 94(3):849–854, 1997.
Meza, D., L. Abejar, D. A. Rubenstein, and W. Yin. A shearing-stretching device that can apply physiological fluid shear stress and cyclic stretch concurrently to endothelial cells. J. Biomech. Eng. 138(3):4032550, 2016.
Meza, D., D. A. Rubenstein, and W. Yin. A comprehensive fluid–structure interaction model of the left coronary artery. J. Biomech. Eng. 140:121006, 2018.
Meza, D., S. K. Shanmugavelayudam, A. Mendoza, C. Sanchez, D. A. Rubenstein, and W. Yin. Platelets modulate endothelial cell response to dynamic shear stress through PECAM-1. Thromb. Res. 150:44–50, 2017.
Mohan, S., N. Mohan, and E. A. Sprague. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. Cell Physiol. 273(2):C572–C578, 1997.
Moore, Jr., J. E., E. Burki, A. Suciu, S. Zhao, M. Burnier, H. R. Brunner, and J. J. Meister. A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann. Biomed. Eng. 22(4):416–422, 1994.
Morrow, D., P. Cullen-John, A. Cahill Paul, and M. Redmond Eileen. Cyclic strain regulates the notch/CBF-1 signaling pathway in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 27(6):1289–1296, 2007.
Nagel, T., N. Resnick, C. F. Dewey, Jr., and M. A. Gimbrone, Jr. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler. Thromb. Vasc. Biol. 19(8):1825–1834, 1999.
Pedrigi, R. M., K. I. Papadimitriou, A. Kondiboyina, S. Sidhu, J. Chau, M. B. Patel, D. C. Baeriswyl, E. M. Drakakis, and R. Krams. Disturbed cyclical stretch of endothelial cells promotes nuclear expression of the pro-atherogenic transcription factor NF-kappaB. Ann. Biomed. Eng. 45(4):898–909, 2017.
Plotnikov, A., E. Zehorai, S. Procaccia, and R. Seger. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 1813(9):1619–1633, 2011.
Potter, C. M. F., S. Schobesberger, M. H. Lundberg, P. D. Weinberg, J. A. Mitchell, and J. Gorelik. Shape and compliance of endothelial cells after shear stress in vitro or from different aortic regions: scanning ion conductance microscopy study. PLoS ONE 7(2):e31228, 2012.
Raaz, U., H. Kuhn, H. Wirtz, and S. Hammerschmidt. Rapamycin reduces high-amplitude, mechanical stretch-induced apoptosis in pulmonary microvascular endothelial cells. Microvasc. Res. 77(3):297–303, 2009.
Remuzzi, A., C. F. Dewey, Jr., P. F. Davies, and M. A. Gimbrone, Jr. Orientation of endothelial cells in shear fields in vitro. Biorheology 21(4):617–630, 1984.
Riou, S., B. Mees, B. Esposito, R. Merval, J. Vilar, D. Stengel, E. Ninio, R. van Haperen, R. de Crom, A. Tedgui, and S. Lehoux. High pressure promotes monocyte adhesion to the vascular wall. Circ. Res. 100(8):1226–1233, 2007.
Rouleau, L., M. Farcas, J. C. Tardif, R. Mongrain, and R. L. Leask. Endothelial cell morphologic response to asymmetric stenosis hemodynamics: effects of spatial wall shear stress gradients. J. Biomech. Eng. 132(8):081013, 2010.
Russo, T. A., D. Stoll, H. B. Nader, and J. L. Dreyfuss. Mechanical stretch implications for vascular endothelial cells: altered extracellular matrix synthesis and remodeling in pathological conditions. Life Sci. 213:214–225, 2018.
Samady, H., P. Eshtehardi, M. C. McDaniel, J. Suo, S. S. Dhawan, C. Maynard, L. H. Timmins, A. A. Quyyumi, and D. P. Giddens. coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation 124(7):779–788, 2011.
Sei, Y. J., S. I. Ahn, T. Virtue, T. Kim, and Y. Kim. Detection of frequency-dependent endothelial response to oscillatory shear stress using a microfluidic transcellular monitor. Sci. Rep. 7(1):10019, 2017.
Seko, Y., N. Takahashi, K. Tobe, T. Kadowaki, and Y. Yazaki. Pulsatile stretch activates mitogen-activated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 259(1):8–14, 1999.
Shanmugavelayudam, S. K., D. A. Rubenstein, and W. Yin. Effects of physiologically relevant dynamic shear stress on platelet complement activation. Platelets 22(8):602–610, 2011.
Stoner, L., J. M. Young, S. Fryer, and M. J. Sabatier. The importance of velocity acceleration to flow-mediated dilation. Int. J. Vasc. Med. 2012:589213, 2012.
Sucosky, P., K. Balachandran, A. Elhammali, H. Jo, and A. P. Yoganathan. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4–and TGF-β1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29(2):254–260, 2009.
Sumpio, B. E., S. Yun, A. C. Cordova, M. Haga, J. Zhang, Y. Koh, and J. A. Madri. MAPKs (ERK½, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J. Biol. Chem. 280(12):11185–11191, 2005.
Thacher, T., V. Gambillara, R. F. da Silva, P. Silacci, and N. Stergiopulos. Reduced cyclic stretch, endothelial dysfunction, and oxidative stress: an ex vivo model. Cardiovasc. Pathol. 19(4):e91–98, 2010.
Tseng, H., T. E. Peterson, and B. C. Berk. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ. Res. 77(5):869–878, 1995.
Tzima, E., M. Irani-Tehrani, W. B. Kiosses, E. Dejana, D. A. Schultz, B. Engelhardt, G. Cao, H. DeLisser, and M. A. Schwartz. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437(7057):426–431, 2005.
Ueki, Y. A., N. Sakamoto, T. Ohashi, and M. Sato. Morphological responses of vascular endothelial cells induced by local stretch transmitted through intercellular junctions. Exp. Mech. 49(1):125, 2009.
van Wolferen, S. A., J. T. Marcus, N. Westerhof, M. D. Spreeuwenberg, K. M. Marques, J. G. Bronzwaer, I. R. Henkens, C. T. Gan, A. Boonstra, P. E. Postmus, and A. Vonk-Noordegraaf. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur. Heart J. 29(1):120–127, 2008.
Walpola, P. L., A. I. Gotlieb, M. I. Cybulsky, and B. L. Langille. Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol. 15(1):2–10, 1995.
Wang, J. G., M. Miyazu, E. Matsushita, M. Sokabe, and K. Naruse. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem. Biophys. Res. Commun. 288(2):356–361, 2001.
White, C. R., H. Y. Stevens, M. Haidekker, and J. A. Frangos. Temporal gradients in shear, but not spatial gradients, stimulate ERK1/2 activation in human endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 289(6):H2350–2355, 2005.
Woodfin, A., M. B. Voisin, and S. Nourshargh. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 27(12):2514–2523, 2007.
Wu, J., S. R. Thabet, A. Kirabo, D. W. Trott, M. A. Saleh, L. Xiao, M. S. Madhur, W. Chen, and D. G. Harrison. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 114(4):616–625, 2014.
Wung, B. S., J. J. Cheng, H. J. Hsieh, Y. J. Shyy, and D. L. Wang. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ. Res. 81(1):1–7, 1997.
Yamamoto, K., and J. Ando. Emerging role of plasma membranes in vascular endothelial mechanosensing. Circ. J. 82(11):2691–2698, 2018.
Yin, W., S. K. Shanmugavelayudam, and D. A. Rubenstein. The effect of physiologically relevant dynamic shear stress on platelet and endothelial cell activation. Thromb. Res. 127(3):235–241, 2011.
Yoshizumi, M., J. Abe, K. Tsuchiya, B. C. Berk, and T. Tamaki. Stress and vascular responses: atheroprotective effect of laminar fluid shear stress in endothelial cells: possible role of mitogen-activated protein kinases. J. Pharmacol. Sci. 91(3):172–176, 2003.
Zhao, S., A. Suciu, T. Ziegler, J. E. Moore, Jr., E. Burki, J. J. Meister, and H. R. Brunner. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler. Thromb. Vasc. Biol. 15(10):1781–1786, 1995.
Funding
This study was in part supported by an American Heart Association Grant in Aid Award (16GRNT30440002).
Conflict of Interest
None for Daphne Meza, Bryan Musmacker, Elisabeth Steadman, Thomas Stransky, David A. Rubenstein, and Wei Yin.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor William H. Guilford oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meza, D., Musmacker, B., Steadman, E. et al. Endothelial Cell Biomechanical Responses are Dependent on Both Fluid Shear Stress and Tensile Strain. Cel. Mol. Bioeng. 12, 311–325 (2019). https://doi.org/10.1007/s12195-019-00585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00585-0