Abstract
Objectives
Due to their widespread presence, persistence, and bioaccumulation in living organisms, microplastics (MP) and methylmercury (MeHg) pollution pose a threat to aquatic organisms. ATP cassette-binding (ABC) protein plays a key role in the efflux of these contaminants from the cell. Although MP and MeHg co-exist in the ocean, their toxicological interaction in marine small crustacean remained to be poorly understood. This study aimed to investigate the combined effects of MP and MeHg on ABC transporter.
Methods
In this study, (1) ABCB and ABCC subfamily genes were identified and characterized in the Diaphanosoma celebensis, (2) the effects of MeHg alone and MeHg + PS MP mixture on ABC transporter activity were examined, and subsequently, (3) the transcriptional modulation of ABCB and ABCC subfamily genes was investigated using quantitative real-time polymerase chain reaction.
Results
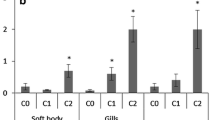
Sequence analysis showed that D. celebensis ABCB and ABCC subfamily genes had conserved domains. Efflux assay revealed that MP inhibited the function of ABCB and ABCC transporter under MeHg co-exposure in a size-dependent manner. The expression of ABCC1-1 and ABCC1-2 was upregulated in the mixture of MeHg and MP higher than MeHg alone.
Conclusions
ABCB and ABCC subfamilies in D. celebensis likely serve as evolutionarily conserved efflux pumps. PS NMPs can enhance the toxicity of MeHg by interrupting the efflux activity of ABC transporters in a particle size-dependent manner.




Similar content being viewed by others
References
Hale RC, Seeley ME, La Guardia MJ, Mai L, Zeng EY (2020) A global perspective on microplastics. J Geophys Res Oceans 125(1):e2018JC014719. https://doi.org/10.1029/2018JC014719
Benson NU, Fred-Ahmadu OH, Bassey DE, Atayero AA (2021) COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. J Environ Chem Eng 9:105222. https://doi.org/10.1016/j.jece.2021.105222
Benson NU, Agboola OD, Fred-Ahmadu OH, De-la-Torre GE, Oluwalana A, Williams AB (2022) Micro(nano)plastics prevalence, food web interactions, and toxicity assessment in aquatic organisms: a review. Front Mar Sci 9:851281. https://doi.org/10.3389/fmars.2022.851281
Birch QT, Potter PM, Pinto PX, Dionysiou DD, Al-Abed SR (2020) Sources, transport, measurement and impact of nano and microplastics in urban watersheds. Rev Environ Sci Biotechnol 19:275–336. https://doi.org/10.1007/s11157-020-09529-x
Dedourge-Geffard O, Palais F, Biagianti-Risbourg S, Geffard O, Geffard A (2009) Effects of metals on feeding rate and digestive enzymes in Gammarus Fossarum: an in situ experiment. Chemosphere 77(11):1569–1576. https://doi.org/10.1016/j.chemosphere.2009.09.042
Jeong CB, Won EJ, Kang HM, Lee MC, Hwang DS, Hwang UK et al (2016) Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ Sci Technol 50(16):8849–8857. https://doi.org/10.1021/acs.est.6b01441
Sıkdokur E, Belivermis M, Sezer N, Pekmez M et al (2020) Effects of microplastics and mercury on manila clam Ruditapes philippinarum: feeding rate, immunomodulation, histopathology and oxidative stress. Environ Pollut 262:114247. https://doi.org/10.1016/j.envpol.2020.114247
Bai Z, Wang N, Wang M (2021) Effects of microplastics on marine copepods. Ecotoxicol Environ Saf 217:112243. https://doi.org/10.1016/j.ecoenv.2021.112243
Lambert S, Wagner M (2018) Microplastics are contaminants of emerging concern in freshwater environments: an overview. In: Wagner M, Lambert S (eds) Freshwater microplastics the handbook of environmental chemistry, 58th edn. Springer, Cham
van Sebille E, Wilcox C, Lebreton L, Maximenko N, Hardesty BD, Van Franeker JA et al (2015) A global inventory of small floating plastic debris. Environ Res Lett 10:124006. https://doi.org/10.1088/1748-9326/10/12/124006
O’Connor D, Hou D, Ok YS, Mulder J, Duan L, Wu Q, Wang S, Tack FMG, Rinklebe J (2019) Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: a critical review. Environ Int 126:747–761. https://doi.org/10.1016/j.envint.2019.03.019
WHO (2017) Chemical aspects. In: WHO (ed) Guidelines for drinking‑water quality, 4th edn. p. 155–200
Al-Sulaiti MM, Soubra L, Al-Ghouti MA (2022) The causes and effects of mercury and methylmercury contamination in the marine environment: a review. Curr Pollution Rep 8:249–272. https://doi.org/10.1007/s40726-022-00226-7
Kehrig HA, Seixas TG, Baêta AP, Malm O, Moreira I (2010) Inorganic and methylmercury: do they transfer along a tropical coastal food web? Mar Pollut Bull 60(12):2350–2356. https://doi.org/10.1016/j.marpolbul.2010.08.010
Reyes-Becerril M, Angulo C, Sanchez V, Cuesta A, Cruz A (2019) Methylmercury, cadmium and arsenic(III)-induced toxicity, oxidative stress and apoptosis in Pacific red snapper leukocytes. Aquat Toxicol 213:105223. https://doi.org/10.1016/j.aquatox.2019.105223
Rosa-Silva HTD, Panzenhagen AC, Schmidtt V, Alves Teixeira A et al (2020) Hepatic and neurobiological effects of foetal and breastfeeding and adulthood exposure to methylmercury in Wistar rats. Chemosphere 244:125400. https://doi.org/10.1016/j.chemosphere.2019.125400
Lee CS, Fisher NS (2017) Bioaccumulation of methylmercury in a marine copepod. Environ Toxicol Chem 36(5):1287–1293. https://doi.org/10.1002/etc.3660
Jang JW, Lee S, Lee B-J, Hur S-W, Son M-H, Kim K-W, Kim K-D, Han H-S (2020) A comparative study of effects of dietary mercuric chloride and methylmercury chloride on growth performance, tissue accumulation, stress and immune responses, and plasma measurements in Korean rockfish, Sebastes schlegeli. Chemosphere 260:127611. https://doi.org/10.1016/j.chemosphere.2020.127611
Kim D-H, Choi B-S, Kang H-M, Park JC, Kim M-S, Hagiwara A, Lee J-S (2021) The genome of the marine water flea Diaphanosoma celebensis: Identification of phase I, II, and III detoxification genes and potential applications in marine molecular ecotoxicology. Comp Biochem Physiol Part D 37:100787. https://doi.org/10.1016/j.cbd.2020.100787
Sturm A, Cunningham P, Dean M (2009) The ABC transporter gene family of Daphnia pulex. BMC Genomics 10:170. https://doi.org/10.1186/1471-2164-10-170
Yu H, Gao Z, Yang Y, Li M, Chen Q (2022) Inhibition of xenobiotics transporters’ efflux ability after nanoplastics exposure in larval Japanese medaka. Water 14(6):863. https://doi.org/10.3390/w14060863
Bridges CC, Zalups RK (2017) Mechanisms involved in the transport of mercuric ions in target tissues. Arch Toxicol 91(1):63–81. https://doi.org/10.1007/s00204-016-1803-y
Straka E, Ellinger I, Balthasar C, Scheinast M et al (2016) Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 340:34–42. https://doi.org/10.1016/j.tox.2015.12.005
Kang HM, Byeon E, Jeong H, Lee Y, Hwang U-K, Jeong C-B, Lee J-S (2021) Arsenic exposure combined with nano-or microplastic induces different effects in the marine rotifer Brachionus plicatilis. Aquat Toxicol 233:105772. https://doi.org/10.1016/j.aquatox.2021.105772
Jeong H, Lee YH, Sayed AEDH, Jeong C-B, Zhou B, Lee J-S, Byeon E (2022) Short- and long-term single and combined effects of microplastics and chromium on the freshwater water flea Daphnia magna. Aquat Toxicol 253:106348. https://doi.org/10.1016/j.aquatox.2022.106348
Marcial HS, Hagiwara A (2007) Multigenerational effects of 17β-estradiol and nonylphenol on euryhaline cladoceran Diaphanosoma celebensis. Fish Sci 73:324–330. https://doi.org/10.1111/j.1444-2906.2007.01338.x
Kim BM, Kang S, Kim RO et al (2018) De novo transcriptome assembly of brackish water flea Diaphanosoma celebensis based on short-term cadmium and benzo[a]pyrene exposure experiments. Hereditas 155:36. https://doi.org/10.1186/s41065-018-0075-3
Bae C, Kim RO, Kim JS et al (2018) Acute toxicity and modulation of an antioxidant defence system in the brackish water flea Diaphanosoma celebensis exposed to cadmium and copper. Toxicol Environ Health Sci 10:186–193. https://doi.org/10.1007/s13530-018-0363-3
In S, Yoon HW, Yoo JW, Cho H, Kim RO, Lee YM (2019) Acute toxicity of bisphenol A and its structural analogues and transcriptional modulation of the ecdysone-mediated pathway in the brackish water flea Diaphanosoma celebensis. Ecotoxicol Environ Saf 179:310–317. https://doi.org/10.1016/j.ecoenv.2019.04.065
Won E-J, Kim D, Yoo J-W, In S, Shin K-H, Lee Y-M (2021) Oxidative stress responses in brackish water flea exposed to microcystin-LR and algal bloom waters from Nakdong river, Republic of Korea. Mar Pollut Bull 162:111868. https://doi.org/10.1016/j.marpolbul.2020.111868
Holland IB, Cole SPC, Kuchler K, Higgins CF (2003) ABC proteins: from bacteria to man. Academic press, London
Dermauw W, Leeuwen V (2014) The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem Mol Biol 45(89):110. https://doi.org/10.1016/j.ibmb.2013.11.001
Jeong CB, Kim BM, Lee JS et al (2014) Genome-wide identification of whole ATP-binding cassette (ABC) transporters in the intertidal copepod Tigriopus japonicus. BMC Genomics 15:651. https://doi.org/10.1186/1471-2164-15-651
Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B (2003) The role of multidrug transporters in drug availability, metabolism and toxicity. Toxicol Lett 140–141:133–143. https://doi.org/10.1016/s0378-4274(02)00497-6
Kim H, Yim B, Kim J, Kim H, Lee Y-M (2017) Molecular characterization of ABC transporters in marine ciliate, Euplotes crassus: identification and response to cadmium and benzo[a]pyrene. Mar Pollut Bull 124(2):725–735. https://doi.org/10.1016/j.marpolbul.2017.01.046
Jeong CB, Kang HM, Lee YH, Kim MS, Lee JS, Seo JS, Wang M, Lee JS (2018) Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer Brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ Sci Technol 52(19):11411–11418. https://doi.org/10.1021/acs.est.8b03211
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301(5637):1203. https://doi.org/10.1126/science.1085941
Lee JS, Oh Y, Park HE, Lee JS, Kim HS (2023) Synergistic toxic mechanisms of microplastics and triclosan via multixenobiotic resistance (MXR) inhibition-mediated autophagy in the freshwater water flea Daphnia magna. Sci Total Environ 896:165214. https://doi.org/10.1016/j.scitotenv
Yoo JW, Choi TJ, Park JS, Kim J, Han S, Kim CB, Lee YM (2023) Pathway-dependent toxic interaction between polystyrene microbeads and methylmercury on the brackish water flea Diaphanosoma celebensis: based on mercury bioaccumulation, cytotoxicity, and transcriptomic analysis. J Hazard Mater 459:132055. https://doi.org/10.1016/j.jhazmat.2023.132055
Xie D, Wei H, Lee J-S, Wang M (2022) Mercury can be transported into marine copepod by polystyrene nanoplastics but is not bioaccumulated: an increased risk? Environ Pollut 303:119170. https://doi.org/10.1016/j.envpol.2022.119170
Yoo J-W, Jeon MJ, Lee K-W, Jung J-H, Jeong C-B, Lee Y-M (2022) The single and combined effects of mercury and polystyrene plastic beads on antioxidant-related systems in the brackish water flea: toxicological interaction depending on mercury species and plastic bead size. Aquat Toxicol 252:106325. https://doi.org/10.1016/j.aquatox.2022.106325
Xie D, Zhang H, Wei H, Lin L, Wang D, Wang M (2023) Nanoplastics potentiate mercury toxicity in a marine copepod under multigenerational exposure. Aquat Toxicol 258:106497. https://doi.org/10.1016/j.aquatox.2023.106497
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165:535–550. https://doi.org/10.1016/j.cell.2016.03.014
Yoo J-W, Cho H, Jeon MJ, Jeong C-B, Jung J-H, Lee Y-M (2021) Effects of polystyrene in the brackish water flea Diaphanosoma celebensis: size-dependent acute toxicity, ingestion, egestion, and antioxidant response. Aquat Toxicol 235:105821. https://doi.org/10.1016/j.aquatox.2021.105821
Sigrist CJA, de Castro E, Cerutti L, Cuche BA et al (2013) New and continuing developments at PROSITE. Nucleic Acids Res 41(D1):D344–D347. https://doi.org/10.1093/nar/gks1067
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Acknowledgements
This work was supported by a grant from Sangmyung University (2023) funded to Young-Mi Lee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Je-Won Yoo, Youn-Ha Lee, Sanghyun Cho, and Young-Mi Lee declare that we have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoo, JW., Lee, YH., Cho, S. et al. Combined effects of microplastics and methylmercury on the activity of ATP-binding cassette (ABC) transporter in the brackish water flea Diaphanosoma celebensis. Toxicol. Environ. Health Sci. 16, 89–98 (2024). https://doi.org/10.1007/s13530-023-00201-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00201-9