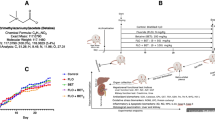
Abstract
Objective
Concerns over fluoride toxicity have increased in recent times due to high exposures from various anthropogenic sources such as industrial sites, fluoride-containing pesticides, drugs, dental products, refridgerants and fire extinguishers. There is, therefore, continued search for agents that could ameliorate the toxicity of this chemical in various body organs. In this study, we sought to investigate the protective effects of L-Arginine (L-Arg), a nitric oxide donor, on liver toxicity induced by sodium fluoride (NaF) in rats.
Methods
Rats received NaF (300 mg L−1) in drinking water alone or in co-treatment with L-Arg at two different doses, 100 and 200 mg kg−1, by oral gavage, for 7 days. Markers of hepatotoxicity, oxidative stress and antioxidant status were thereafter assessed.
Results
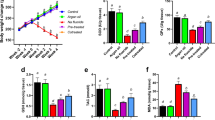
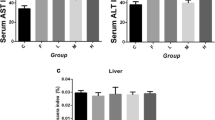
NaF caused marked increase in serum transaminases: alanine aminotransferase, Aspartate aminotransferase and Alkaline phosphatase, along with atrophy of the centri-lobular hepatic cords and dilatation of the sinusoids. Moreover, NaF stimulated increases in hepatic contents of hydrogen peroxide (H2O2), nitric oxide (NO), protein carbonyls, malondialdehyde and advanced oxidation protein products. NaF also inhibited the activities of antioxidant enzymes, Glutathione peroxidase and Superoxide dismutase. However, L-Arg supplementation caused significant alleviation of NaF hepatotoxicity by reducing lipid and protein oxidation indices, stimulation of antioxidant systems along with increased production of NO.
Conclusions
L-Arg showed promise as a potential protective agent against NaF-induced hepatotoxicity via restoration of oxidant-antioxidant balance. Further studies are required to understand the involvement of NO signaling in the protective effects of L-Arg against fluoride toxicity.




taken from the liver of control rats treated with distilled water only and shows normal appearance of hepatic architecture, with no visible lesions. Plate B shows a section taken from the liver of rats exposed to sodium fluoride alone, with the major lesions comprising atrophy of the centrilobular hepatic cords and accompanying sinusoidal dilatation and enlarged central vein (encircled), giving a generalized “mosaic” pattern of the liver parenchyma. Plates C and D represent liver sections taken from rats treated with L-Arg at 100 and 200 mg kg−1, respectively, along with NaF exposure. These sections showed no visible lesions
Similar content being viewed by others
References
Harrison PT (2005) Fluoride in water: a UK perspective. J Fluoride Chem 126:1448–1456
Kabir H, Gupta AK, Tripathy S (2019) Fluoride and human health: systematic appraisal of sources, exposures, metabolism and toxicity. Crit Revs Environ Sci Technol 50(11):1116–1193
World Health Organization (1996) Trace elements in human nutrition and health. WHO, Geneva
Atmaca N, Atmaca HT, Kanici A et al (2014) Protective effect of resveratrol on sodium fluoride-induced oxidative stress, hepatotoxicity and neurotoxicity in rats. Food Chem Toxicol 70:191–197
Hamza RZ, El-Shenawy NS, Ismail HA (2015) Protective effects of blackberry and quercetin on sodium fluoride-induced oxidative stress, histological changes in the hepatic, renal, testis and brain tissues of male rat. J Basic Clin Physiol Pharmacol 26(3):237–251
Ullah R, Zafar MS, Shahani N (2017) Potential fluoride toxicity from oral medicaments: a review. Iran J Basic Med Sci 20(8):841–848
Garcia-Montalvo EA, Reyes-Perez H, Del Razo LM (2009) Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology 263:75–83
Ghosh J, Das J, Manna P et al (2008) Cytoprotective effect of arjunolic acid in response to sodium fluoride-mediated oxidative stress and cell death via necrotic pathway. Toxicol In Vitro 22:1918–1926
Shivarajashankara YM, Shivashankara AR, Gopalakrishna BP et al (2001) Oxidative stress in children with endemic skeletal fluorosis. Fluoride 34:108–113
Di Pierro F, Rossoni G (2013) An amino acids mixture improves the hepatotoxicity induced by acetaminophen in mice. J Amino Acids. https://doi.org/10.1155/2013/61574
Kirk SJ, Barbul A (1990) Role of arginine in trauma, sepsis and immunity. JPEN J Parenter Enteral Nutr 14:226S
Leeuwenburgh C, Heinecke JW (2001) Oxidative stress and antioxidants in exercise. Curr Med Chem 8:829–838
Bailey SJ, Winyard PG, Vanhatalo A et al (2010) Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J Appl Physiol 109:1394–1403
Nisoli E, Falcone S, Tonello C et al (2004) Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101:16507–16512
Saad E (2012) Curative and protective effects of L-arginine on carbon tetrachloride-induced hepatotoxicity in mice. Biochem Biophys Res Commun 423:147–151
Saleh S, El-Demerdash E (2005) Protective effects of L-Arginine against cisplatin-induced renal oxidative stress and toxicity: role of nitric oxide. Basic Clin Pharmacol Toxicol 97:91–97
Bijle MNA, Ekambaram M, Lo ECM, Yiu CKY (2019) The combined antimicrobial effect of arginine and fluoride toothpaste. Sci Rep 9:8405
Yamada J, Tomiyama H, Yambe M et al (2006) Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atheroscler 189(1):198–205
Ischiropoulos H, Al-Mehdi AB (1995) Peroxynitrite-mediated oxidative protein modifications. FEBS Lett 364(3):279–282
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidat Med Cell Longev. https://doi.org/10.1155/2014/360438
Scott GS, Bolton C (2000) L-Arginine modifies free radical production and the development of experimental allergic encephalomyelitis. Inflamm Res 49:720–726
Hassan HA, Yousef MI (2009) Mitigating effects of antioxidant properties of blackberry juice on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol 47:2332–2337
Knowles RG, Moncada S (1994) Nitric oxide synthase in mammals. Biochem J 298(Pt 2):249–258
Xia Y, Tsai AL, Berka V et al (1998) Superoxide generation from endothelial nitric oxide synthase. A C2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273:25804–25808
Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24:413–420
Vesna O, Biljana B, Aleksandra K et al (2009) L-arginine supplementation induces glutathione synthesis in interscapular brown tissue adipose tissue through activation of glutamate-cysteine ligase expression: the role of nitric oxide. Chemico-Biol Interact 182(2–3):204–212
Vasant RA, Narasimhacharya AVRI (2012) Ameliorative effect of Tamarind Leaf on fluoride-induced metabolic alterations. Environ Health Prev Med 17:484–489
Bracatelli G, Furlan A, Calandra A et al (2018) Hepatic sinusoidal dilatation. Abdom Radiol 43(8):2011–2022
Pai RK, Hart JA (2010) Aberrant expression of cytokeratin 7 in perivascular hepatocytes correlates with a cholestatic chemistry profile in patients with heart failure. Modern Pathol 23:1650–1656
Akinrinde AS, Soetan KO, Tijani MO (2020) Exacerbation of diclofenac-induced gastroenterohepatic damage by concomitant exposure to sodium fluoride in rats: protective role of luteolin. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2020.1802478
Reitmann S, Frankel S (1957) Colorimetric method for the determination of serum transaminase activity. Am J Clin Pathol 28:56–68
Gscc R (1972) Optimised standard colorimetric methods. J Clin Chem Clin Biochem 10:182
Gornal AG, Bardawill JC, David MM (1949) Determination of serum proteins by means of biuret reaction. J Biol Chem 177:751–766
Wolff SF (1994) Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxides. Methods Enzymol 233:182–189
Olaleye SB, Adaramoye OA, Erigbali PP et al (2007) Lead exposure increases oxidative stress in the gastric mucosa of HCl/-ethanol-exposed rats. World J Gastroenterol 13(38):5121–5126
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Kayali R, Cakatay U, Akcay T et al (2006) Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct 24:79–85
Varshney R, Kale RK (1990) Effect of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58:733–743
Jollow DJ, Mitchell JR, Zampaglione N (1974) Bromobenzene-induced liver necrosis: protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11(3):151–169
Ellman GL (1959) Tissue sulfhydryl groups. Archiv Biochem Biophys 82:70–77
Rotruck JT, Pope AL, Ganther HE et al (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 25:7130–7139
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Drury RA, Wallington EA, Cancerson R (1976) Carlton’s histopathological techniques, 4th edn. Oxford University Press, Oxford
Acknowledgements
The authors wish to acknowledge the technical assistance provided by Mr. O. Agboola of the Department of Veterinary Physiology and Biochemistry, University of Ibadan.
Author information
Authors and Affiliations
Contributions
AAS and AAO contributed to the conceptualization and design of the study; AAS and MT did the analysis and interpretation of the data; AAS wrote the first draft of the manuscript; AAS and AAO did the critical revision of the manuscript. AAS, MT and OAA carried out the experimental assays.
Corresponding author
Ethics declarations
Conflict of interest
Akinleye Akinrinde, Monsuru Tijani, Olusola Awodele and Ademola Oyagbemi declare that they have no conflict of interest.
Ethical approval
The study was conducted following guidelines approved by the Animal Care and Use Research Ethics Committee (ACUREC) of the University of Ibadan (Approval number: UI-ACUREC/19/124). The conduct of the experiments, humane animal handling and welfare was guided by protocols contained in the publication of the National Institute of Health, “Guide for the Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Akinrinde, A.S., Tijani, M., Awodele, O.A. et al. Fluoride-induced hepatotoxicity is prevented by L-Arginine supplementation via suppression of oxidative stress and stimulation of nitric oxide production in rats. Toxicol. Environ. Health Sci. 13, 57–64 (2021). https://doi.org/10.1007/s13530-020-00070-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-020-00070-6