Abstract
Purpose
This study assessed the effectiveness of basal-bolus insulin therapy (BBIT) in non-critically ill patients with type 2 diabetes mellitus (DM) and the factors associated with optimal inpatient glycemic control (IGC) with BBIT.
Methods
This prospective study included 103 patients who were admitted to the University Medical Center and were treated with BBIT. Clinical characteristics, glucose, and glycated hemoglobin (HbA1c) levels at admission, renal function tests, basal-bolus insulin dosing, and other treatments were recorded. The optimal IGC was defined and classified for the analysis.
Results
The mean age of the patients was 67.2 ± 12.0 years. The blood glucose and HbA1c levels at admission were 319.2 ± 184.8 mg/dL and 10.7 ± 2.6%, respectively. Optimal IGC was defined as patients with ≥60% of in-hospital blood glucose values within the target range (3.9–10 mmol/L). Of the 103 patients, 66 patients (64%) achieved optimal IGC and only 5 patients (4.9%) had at least one hypoglycemic episode. The number of patients consuming snacks was higher in the poor than in the optimal IGC group whereas an estimated glomerular filtration rate (eGFR) <45-mL/min/1.73 m2 was predominant in the optimal IGC group. Multivariate analysis revealed that snack consumption and glucocorticoid (GC) use were factors associated with poor IGC, while eGFR <45 mL/min/1.73 m2 was a favorable factor for optimal IGC.
Conclusion
BBIT is safe and effective for the treatment of IGC in non-critically ill patients. Moreover, eGFR <45 mL/min/1.73 m2 at admission, snack consumption, and GC therapy were independent factors associated with IGC outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients diagnosed with diabetes mellitus (DM) have a three-fold higher rate of hospitalization than those without DM [1]. Hyperglycemia (blood glucose [BG] level > 140 mg/dL [7.8 mmol/L]) has been reported in 22–46% of non-critically ill-hospitalized patients [2, 3]. Inpatient hyperglycemia is associated with increased morbidity and mortality, longer hospital stay, higher transfer rate to the intensive care unit, and greater need for transitional or nursing home care after discharge [3].
Insulin regimens are important for inpatient glycemic control (IGC) in non-critically ill patients with type 2 DM [2, 4]. Subcutaneous insulin therapy, which comprises a basal long-acting insulin dose (glargine, detemir, or degludec) once daily or an intermediate-acting insulin (neutral protamine Hagedorn) twice daily alone or in combination with short- (regular) or rapid-acting insulin (lispro, aspart, or glulisine) before meals, is preferred for IGC in these patients [2, 5]. This combination regimen results in better BG levels [6,7,8], lower frequency of hypoglycemic events [9], and fewer hospital complications [7] than other insulin regimens. This regimen, also called basal-bolus insulin therapy (BBIT), is the potential treatment of choice for maintaining BG levels in the range of 6.1–10 mmol/L in non-critically ill patients.
Although BBIT has been studied and used worldwide for over a decade and its effectiveness is universally accepted, it has not yet been widely applied in Vietnamese hospitals. In many Asian countries in general, and Vietnam in particular, premixed insulin is still predominantly used for inpatient glycemic control [10, 11]. BBIT has gained popularity over the last 5 years in some large medical centers. Therefore, no report on BBIT outcomes has been published in Vietnam. To date, there are recommendations only for the BG target for inpatient hyperglycemia management, but no criteria are available for optimal IGC in any guidelines or consensus, especially when the BBIT was utilized [2]. In practice, the threshold for optimal IGC is often chosen by each author. In our own experience, optimal IGC was confirmed if patients had more than or equal to 60% of their in-hospital BG values falling within the target range (3.9–10 mmol/L). We aimed to determine the effectiveness of BBIT using a new definition of optimal IGC in non-critically ill patients with type 2 DM in Vietnam.
In general, IGC seems to be significantly associated with preadmission diabetes control (HbA1c and admission BG) and inpatient glucocorticoid use [12, 13]. However, there are not many studies on the predictors of IGC when using BBIT in non-critically ill patients with type 2 DM both worldwide and in Vietnam.
Hence, we conducted this study to assess the effectiveness of BBIT with a new definition of optimal IGC and to identify the factors that affect IGC in Vietnamese patients with type 2 DM in a non-intensive care setting.
Materials and methods
Study design
This prospective study was approved by the University of Medicine and Pharmacy at Ho Chi Minh city Ethics Committee on October 31, 2019, and conducted from November 2019 to June 2020 at the Endocrinology Department of the University Medical Center (UMC) in Ho Chi Minh City, Vietnam.
Patients aged ≥18 years were enrolled if type 2 DM diagnosis was confirmed and they had been previously treated with diet management, hypoglycemic agents, and insulin. All patients had experienced a hospital stay of at least 6 days. The exclusion criteria included type 1 DM diagnosis, pregnancy, acute hyperglycemic emergencies (diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome), severe hyperglycemia requiring intravenous insulin infusion on admission, continuous enteral and parenteral nutrition, change in nutritional support during the study period, hypoglycemia on admission, surgical interventions, ongoing corticosteroid therapy (>40 mg/day of equivalent hydrocortisone), and mental health conditions which prevented patients from providing informed consent (Fig. 1).
Procedures
The patients were initially treated with BBIT according to an institutionally approved protocol on admission (Appendix). All oral hypoglycemic agents, including metformin and dipeptidyl peptidase-4 inhibitors, were discontinued. The daily insulin dose (DID) was based on the total DID before admission or the patient’s body weight. BBIT was administered subcutaneously as follows:
Patients treated with insulin before admission were administered their previous total DID.
The starting DID for patients treated with diet management or oral hypoglycemic agents before admission was as follows.
-
Insulin-sensitive (thin stature, poor appetite, elderly, presence of chronic kidney disease, and no history of insulin treatment): 0.3 units/kg
-
Insulin-resistant (obese, presence of infection, ongoing glucocorticoid [GC] treatment): 0.5–1 units/kg
-
No characteristics of insulin sensitivity/resistance: 0.4 units/kg
Half of the DID was prescribed as basal insulin glargine (100 units/mL; LANTUS® SoloStar®, Sanofi S.A., Paris, France) once daily at the same time. The other half was prescribed regular insulin (100 units/mL; Actrapid® HM, Novo Nordisk A/S, Bagsværd, Denmark), divided into equal doses administered before meals. Preprandial rapid-acting insulin was administered until oral food intake could prevent hypoglycemia in patients who were unable to eat. Patients received supplemental (correction) doses of insulin before meals and at bedtime, according to a previously used protocol (Appendix).
Capillary BG was measured four times a day if the patients could consume three meals per day: fasting, 5 a.m.–6 a.m.; preprandial, 10 a.m.–11 a.m.; afternoon, 4 p.m.–5 p.m.; and postprandial or at bedtime, around 9 p.m.–10 p.m. Pre-meal capillary BG level was measured in patients receiving supported enteral nutrition. The BG test was performed using a point-of-care glucose meter (AccuChek Performa, Roche Diagnostics, Basel, Switzerland). Additionally, BG was checked whenever a patient showed signs of hypoglycemia, according to the Endocrine Society 2012 guidelines [2]. Laboratory tests, including basic biochemical studies, lipid profiles, and HbA1c tests, were conducted for all patients on the first day.
Capillary BG of the patients was monitored for at least 4 days and up to 6 days after admission. The capillary BG levels recorded on the first day after admission (day 0) were excluded from the dataset because of multiple confounding factors that might affect BG concentration on admission, such as stress, acute illnesses, decompensated conditions of chronic diseases, changes in nutritional support, and pre-hospitalized medical treatments. Day 0 represents an unstable period of BG control and might not reflect the quality of inpatient care. Moreover, a supplemental (correction) insulin dose was not administered on day 0. Capillary BG levels on the second day (day 1) were included in the analyses.
Definition of variables
The target BG level in the range of 3.9–10 mmol/L was defined as the optimal IGC.
Hypoglycemia and hyperglycemia were defined as BG < 3.9 mmol/L and > 10.0 mmol/L, respectively. Severe hypoglycemia was defined as the loss of consciousness and/or seizures.
BBIT effectiveness was assessed using a “patient model.” It was an analytic model that employed all the BG values of a patient collected during their hospitalization, except for day 0, to calculate the following parameters: mean BG measurement during each patient’s in-hospital stay, percentage of BG values within the optimal range (3.9–10 mmol/L), and percentage of BG values < 3.9 mmol/L and >10.0 mmol/L. In another model, the “patient-day model,” the BG values of each patient were categorized according to the calendar days. The mean patient-day glucose level was calculated as the mean BG per day. The percentage of BG values between 3.9 mmol/L and 10.0 mmol/L per patient per day was also included. These events were confirmed by a time point fingerstick BG monitoring method rather than using the mean values.
Patients were divided into optimal and poor IGC groups after receiving BBIT. The former included patients with ≥60% of their in-hospital BG values within the target range (3.9–10 mmol/L).
To assess the factors associated with IGC in patients treated with BBIT, we analyzed factors including the duration of diabetes, HbA1c, admission blood glucose, infection, high waist circumference, estimated glomerular filtration rate, inpatient glucocorticoid therapy, and snack consumption.
A snack in a hospital is any carbohydrate-containing meal that is not part of the “in-hospital diet” formulated by dieticians and endocrinologists. Snack consumption was confirmed by caregiver observations or patient statements during in-hospital care. Snack consumption was defined as consumption of snacks for more than 40% of the total follow-up days in the hospital.
Inpatient glucocorticoid therapy was defined as an inpatient receiving glucocorticoid therapy for more than 40% of the total follow-up days in the hospital.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range) depending on the data distribution. Categorical variables were presented as frequencies and percentages. The Kolmogorov–Smirnov test was used to determine the normality of the distributions. Comparisons between continuous variables were performed using two-tailed independent Student’s t-test or Mann–Whitney U test. Categorical variables were analyzed using chi-square tests with Yates correction or Fisher’s exact test. Logistic regression was performed to explore independent risk factors associated with poor IGC.
All statistical analyses were performed using STATA IC 14 (StataCorp LLC, College Station, TX, USA); p-values < 0.05 were considered statistically significant.
Results
Table 1 presents the demographic characteristics. The mean age of enrolled patients was 67.2 ± 12.0 years. The mean duration since DM diagnosis was 12.5 ± 9.4 years. Nearly two-thirds of the patients were overweight or obese (body mass index [BMI] ≥ 23 kg/m2 for Asians), with an average BMI of 24 kg/m2. Patients using insulin before hospitalization accounted for 62.1% of the sample, and premixed insulin therapy was the most common treatment (49.5%). The BG and HbA1c levels at admission were 319.2 ± 184.8 mg/dL and 10.7 ± 2.6%, respectively. Infectious diseases in 68 patients (66%) were the main diagnoses during admission.
Glycemic control
The mean BG level in this patient model was 10.7 ± 3.1 mmol/L. The percentage distribution of BG values < 3.9 mmol/L, 3.9–10.0 mmol/L, and > 10.0 mmol/L were 0.3 ± 1.4%, 57.3 ± 31.5%, and 42.4 ± 31.8%, respectively (Table 1).
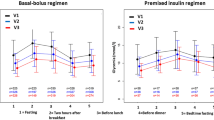
The patient-day model revealed each patient’s BG level on the calendar day. The daily BG levels showed a downward trend, wherein the BG level on day 3 was significantly lower than that of day 0 (10.4 ± 3.1 vs. 12.5 ± 3.6 mmol/L; p < 0.001) (Fig. 2). However, the percentage of BG values within the target range on day 3 was higher than that on day 0 (60.6 ± 36.3% vs. 34.9 ± 36.1%; p <0.001) (Fig. 3).
Changes in the mean daily percentage of blood glucose values in the range of 3.9–10.0 mmol/L. The percentage of BG values in the range of 3.9–10.0 mmol/L showed an upward trend, wherein the percentage of BG within the target range on day 3 was higher than that on day 0 (60.6 ± 36.3% vs. 34.9 ± 36.1%; p <0.001). BG: blood glucose
Regarding BG control, 64.1% (66/103) of the patients presented optimal IGC. Additionally, 4.9% (5/103) of the patients had at least one episode of hypoglycemia during hospitalization, and all of them belonged to the optimal IGC group (7.6%). No hypoglycemic cases were observed in the poor IGC group. Moreover, no severe hypoglycemia-related complications (unconsciousness or seizures) were observed in either group.
Differences between Optimal IGC and Poor IGC groups
The optimal and poor IGC groups included 66 and 37 patients, respectively (Fig. 1).
The BG and HbA1c levels on admission did not differ statistically between the two groups (p = 0.33 and p = 0.11, respectively) (Table 1). The percentage of patients with estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 was significantly lower (13.5% vs. 36.4%, p = 0.01) and that of patients consuming snacks was four-fold higher (67.6% vs. 15.2%, p < 0.001) in the poor IGC group than in the optimal IGC group. According to the optimal IGC subgroups’ analysis, patients with eGFR < 45 mL/min/1.73 m2 required lower DID than those with eGFR ≥ 45 mL/min/1.73 m2 (0.74 ± 0.04 vs. 0.65 ± 0.05 units/kg/day; p = 0.21). Inpatients received nutritional support or diabetes-care education equally, regardless of group (p = 0.416 and p = 1.000, respectively). Regarding the use of GCs, the poor IGC patients outnumbered the optimal IGC patients (40.5% vs. 25.8%), but without a statistically significant difference in the univariate analysis (p = 0.12). However, in the multivariate analysis, the GC use was significantly associated with the poor IGC outcomes (odds ratio [OR] = 2.6, 95% confidence interval, 1.0–6.8; p = 0.048) (Table 2).
Regarding insulin administration, patients with poor IGC required a higher total DID, basal DID, bolus DID, and DID (units/kg/day) than the optimal IGC group, but the difference was not statistically significant (p > 0.05) (Table 1). Furthermore, the percentage of bolus DID was higher and that of basal DID was lower in the poor IGC group than in the optimal IGC group (p = 0.04) (Table 1).
Discussion
Despite its worldwide application in recent decades, BBIT has only been introduced in Vietnamese healthcare centers recently. UMC is one of the few hospitals in which this insulin regimen has been applied for inpatient treatment. Thus, this is a pioneering study on the effectiveness of BBIT and the factors associated with IGC when using this regimen in non-critically ill patients with type 2 DM in Vietnam.
The principal standard of effective glycemic treatment is the outcome of IGC. Previous researchers have selected various thresholds to determine IGC. Goldberg et al. set a very high cutoff (≥ 85% of BG values should be in the target range) to qualify as optimal IGC [14]. Pasquel et al. included those patients with approximately 60% of BG values in the 3.9–10.0 mmol/L range in the IGC group [15]. Our center chose a cutoff of 60% of BG values within the target range to qualify for the optimal IGC group. In our opinion, this threshold is not too high to accomplish, and is a reasonable treatment goal. In clinical practice, there are recommendations only for the BG target for inpatient hyperglycemia management, whereas there are no criteria available for optimal IGC in any guidelines or consensus [2]. Thus, the IGC cutoff depends mainly on the experience of each research team.
Based on our definition of optimal IGC, 64.1% of our patients achieved optimal IGC during hospitalization, of whom more than half had no hypoglycemic events. The Randomized Study of BBIT (RABBIT 2) trial reported 66% of BBIT-induced patients had optimal BG levels (< 140 mg/dL) [8]. There was little difference in the optimal IGC results between the RABBIT 2 trial and our study, except for the optimal IGC definition. The RABBIT 2 trial compressed each patient’s BG values during the entire hospital stay into a single mean value. This maneuver did not reflect the BG variability throughout the follow-up period and underestimated the relative impact of the extended length of stay on the patient’s outcomes. In contrast, our research used the “patient model” to calculate the percentage of in-hospital BG values for each patient in the target range. This model provided a balanced approach that attributed a patient’s IGC to each BG value, BG monitoring frequency, and length of stay. The IGC outcomes in our study seemed to be better than the outcomes in Pérez’s study, which demonstrated that 47.7% of the patients achieved a fasting BG between 90 and 130 mg/dL, and 30.2% presented postprandial BG ≤ 180 mg/dL [16].
In our study, 4.9% of the patients had at least one hypoglycemic episode, which was not very high as compared to the 3% recorded in the RABBIT 2 trial (hypoglycemic cutoff < 60 mg/dL) [8]. Zaman Huri et al. presented similar results: 2.5% of patients had hypoglycemia (< 60 mg/dL) and 5.7% of patients had BG levels < 70 mg/dL [17]. The variation of “optimal” BG outcomes and hypoglycemic rates could be attributed to the different optimal IGC definitions and the different hypoglycemic cutoffs used in certain studies. Regardless of the hypoglycemic cutoff, the incidence of hypoglycemia was low in BBIT-treated populations. This conclusion is similar to that of previous BBIT studies. Based on our institutional BBIT protocol, regular BG monitoring and correction of bolus insulin dose before each meal contributed to reducing the hypoglycemic incidence in patients with poor appetite (see Appendix). The low incidence of hypoglycemia in our department could be attributed to many factors. First, the fear of hypoglycemia seemed to be a major barrier to improving IGC. Physicians tend to prescribe lower doses of starting insulin and correction doses to ensure that their patients do not experience a hypoglycemic event. Second, up to one third of the study population consumed snacks during their hospital stay, although it was not recommended to consume additional food between the main repasts. Snack consumption protected DM patients from hypoglycemia but negatively affected IGC. Finally, patients might have had asymptomatic hypoglycemia during periods when capillary BG levels were not checked. Such hypoglycemic episodes can be detected through continuous glucose monitoring.
Similar to other Asian population studies, such as the study by Yamamoto et al [18], our study proved that bolus DID accounted for the majority of the total DID. Such a high bolus DID is derived from the adjustment of the pre-prandial DID. This insulin dose was often higher than the basal DID if we refer to the guideline for BBIT initiation that recommends a 1:1 basal-to-bolus ratio. The high bolus DID could be attributed to elevated postprandial glycemia owing to the carbohydrate-rich ingredients used in Asian recipes.
Multivariate analyses showed that there were some independent factors associated with IGC outcomes in BBIT-treated patients, including eGFR < 45 mL/min/1.73 m2 (p = 0.026), in-hospital snack consumption (p < 0.001), and GC use (p = 0.048). These outcomes nearly matched those of the univariate analyses, wherein snack consumption was a predictive factor associated with poor IGC, while eGFR < 45 mL/min/1.73 m2 was a favorable factor for optimal IGC. In patients diagnosed with diabetic kidney disease (DKD), a large proportion of exogenous insulin (30–80%) is metabolized and degraded by the kidneys as compared to the non-DKD patients [19]. Therefore, it is necessary to decrease the total DID to avoid hypoglycemia in these patients [20, 21]. Type-2 DM patients with reduced eGFR tend to achieve the BG target with a lower total DID than those with normal eGFR (0.65 ± 0.05 vs. 0.74 ± 0.04 units/kg/day; p = 0.21).
Furthermore, the use of GCs, considered ineffective for IGC in univariate analysis (p = 0.120), was a notable predictor of poor IGC in multivariate analysis. The use of GCs in patients with pre-existing diabetes will undoubtedly worsen IGC [11]. Another study with a large number of participants showed that GC use was an important predictor of poor IGC [11]. GCs aggravate hyperglycemia in patients with known DM [22], leading to a hyperglycemia incidence of up to 46% and an increase in the BG levels by approximately 68% as compared to the baseline values [23, 24]. Additionally, there can be acute complications such as non-ketotic hyperosmolar state, diabetic ketoacidosis [25], and even death in patients with pre-existing DM.
Our study had some limitations. First, this study was merely a descriptive study that introduced a new insulin regimen (BBIT) and analyzed IGC outcomes in hospitalized Vietnamese patients. As a pilot study, we lacked a control group that used other insulin regimens for comparison with BBIT. Second, the correction of bolus insulin before meals depended only on capillary BG levels because there were no ingredient analytic platforms (especially carbohydrate estimation) assessing inpatients’ meals. Finally, the study did not investigate the impact of BBIT on long-term outcomes, including length of stay, diabetes-related complications, or mortality. We have not yet performed a follow-up after discharge to evaluate the outpatient glycemic control for mid- and long-term periods, which is an essential evidence to not only calibrate a safe BBIT dosage but also ensure crucial surveillance of homecare patients.
Conclusion
In conclusion, type-2 DM inpatients who received BBIT achieved a high optimal IGC with acceptable hypoglycemic rates in a noncritical care department. Supplementary meals, especially snacks, and GC therapy were factors associated with poor IGC, whereas decreased eGFR was identified as a favorable factor for optimal IGC when using BBIT to manage in-hospital hyperglycemia. Further studies on factors associated with glycemic control with BBIT should be conducted in non-critically ill patients with type 2 DM, especially homecare patients.
Data availability
Not applicable.
References
Jiang HJ, Stryer D, Friedman B, Andrews R. Multiple hospitalizations for patients with diabetes. Diabetes Care. 2003;26(5):1421–6.
Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, van den Berghe G. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82.
Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE, American Association of Clinical Endocrinologists, American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31.
King AB, Armstrong DU. Basal bolus dosing: a clinical experience. Curr Diabetes Rev. 2005;1(2):215–20.
Umpierrez GE, Smiley D, Hermayer K, Khan A, Olson DE, Newton C, Jacobs S, Rizzo M, Peng L, Reyes D, Pinzon I, Fereira ME, Hunt V, Gore A, Toyoshima MT, Fonseca VA. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–74.
Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–61.
Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, Puig A, Mejia R. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–6.
Bellido V, Suarez L, Rodriguez MG, Sanchez C, Dieguez M, Riestra M, Casal F, Delgado E, Menendez E, Umpierrez GE. Comparison of Basal-Bolus and Premixed Insulin Regimens in Hospitalized Patients With Type 2 Diabetes. Diabetes Care. 2015;38(12):2211–6.
Sheu WH, Ji L, Lee WJ, Jabbar A, Han JH, Lew T. Efficacy and safety of premixed insulin analogs in Asian patients with type 2 diabetes: A systematic review. J Diabetes Investig. 2017;8(4):518–34. https://doi.org/10.1111/jdi.12605.
Nguyen KT, Diep BTT, Nguyen VDK, Van Lam H, Tran KQ, Tran NQ. A cross-sectional study to evaluate diabetes management, control and complications in 1631 patients with type 2 diabetes mellitus in Vietnam (DiabCare Asia). Int J Diabetes Dev Ctries. 2020;40:70–9. https://doi.org/10.1007/s13410-019-00755-w.
Bender M, Smith TC, Thompson J, Koucheki A, Holdy K. Predictors of Suboptimal Glycemic Control for Hospitalized Patients with Diabetes: Targets for Clinical Action. J Clin Outcomes Manag. 2015;22(4):159–67.
Alaqil A, Alkahtani M, Algahtani M. Prevalence and Predictors of Glycemic Control in Hospitalized Patients with Diabetes. Indian J Public Health Res Dev. 2020;48(11):1449–53.
Goldberg PA, Bozzo JE, Thomas PG, Mesmer MM, Sakharova OV, Radford MJ, Inzucchi SE. Glucometrics-assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8(5):560–9.
Pasquel FJ, Gianchandani R, Rubin DJ, Dungan KM, Anzola I, Gomez PC, Peng L, Hodish I, Bodnar T, Wesorick D, Balakrishnan V, Osei K, Umpierrez GE. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–33.
Perez A, Reales P, Barahona MJ, Romero MG, Minambres I, Group H S. Efficacy and feasibility of basal-bolus insulin regimens and a discharge-strategy in hospitalised patients with type 2 diabetes-the HOSMIDIA study. Int J Clin Pract. 2014;68(10):1264–71.
Zaman Huri H, Permalu V, Vethakkan SR. Sliding-scale versus basal-bolus insulin in the management of severe or acute hyperglycemia in type 2 diabetes patients: a retrospective study. PLoS One. 2014;9(9):e106505.
Yamamoto S, Hayashi T, Ohara M, Goto S, Sato J, Nagaike H, Fukase A, Sato N, Hiromura M, Tomoyasu M, Nakanishi N, Lee S, Osamura A, Yamamoto T, Fukui T, Hirano T. Comparison of liraglutide plus basal insulin and basal-bolus insulin therapy (BBIT) for glycemic control, body weight stability, and treatment satisfaction in patients treated using BBIT for type 2 diabetes without severe insulin deficiency: A randomized prospective pilot study. Diabetes Res Clin Pract. 2018;140:339–46.
Rajput R, Sinha B, Majumdar S, Shunmugavelu M, Bajaj S. Consensus statement on insulin therapy in chronic kidney disease. Diabetes Res Clin Pract. 2017;127:10–20.
Charpentier G, Riveline JP, Varroud-Vial M. Management of drugs affecting blood glucose in diabetic patients with renal failure. Diabetes Metab. 2000;26(Suppl 4):73–85.
Snyder RW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin Dial. 2004;17(5):365–70.
Trence DL. Management of patients on chronic glucocorticoid therapy: an endocrine perspective. Prim Care. 2003;30(3):593–605.
Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12(4):358–62.
Tamez-Perez HE, Quintanilla-Flores DL, Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Tamez-Pena AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes. 2015;6(8):1073–81.
Cagdas DN, Pac FA, Cakal E. Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther. 2008;13(4):298–300.
Acknowledgements
We would like to express our gratitude to the medical staff of the Endocrinology Department of University Medical Center, Ho Chi Minh City, for their support in data collection.
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Mai Ngoc Thi Tran, Nam Quang Tran, and Khiem Tran Dang. The first draft of the manuscript was written by Mai Ngoc Thi Tran and all authors thoroughly commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Council for Medical Research of the University of Medicine and Pharmacy at Ho Chi Minh City (Protocol number: 584, University of Medicine and Pharmacy, BOARD, October 31, 2019).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors have consented to have their data published in a journal article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Protocol identifying number: code 584/ University of Medicine and Pharmacy – BOARD, October 31, 2019.
Supplementary Information
ESM 1
(DOCX 21 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, M.N.T., Dang, K.T., Ly, L.D. et al. Basal-bolus insulin therapy for the treatment of non-critically ill patients with type 2 diabetes in Vietnam: effectiveness and factors associated with inpatient glycemic control. Int J Diabetes Dev Ctries 43, 199–207 (2023). https://doi.org/10.1007/s13410-022-01079-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-022-01079-y