Abstract
Purpose
The role of cytokine-producing B cells in antitumor immunity is mostly overlooked. In the present study, we investigated changes in B cell cytokine profiles in breast tumor-draining lymph nodes (TDLNs) during disease progression, and associations of these changes with prognostic indicators.
Methods
Flow cytometry was used to measure the expression of TNF-α, IL-10, TGF-β, IL-2 and IFN-γ in B cells from 42 axillary lymph nodes. The frequencies of IL-10+ and FoxP3+ T regulatory cells (Tregs) were also determined.
Results
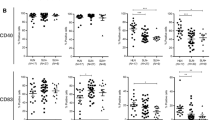
No significant changes in B cell cytokine profiles were observed during breast cancer progression from stage I to III, but the percentage of B cells with high TNF-α expression (TNFhi) showed a negative relationship with lymph node involvement and Her2 expression (p < 0.05). The percentage of IL-10+ B cells was found to be significantly higher in non-metastatic lymph nodes in node-negative compared to node-positive patients (p = 0.001). The frequencies of IL-10+ and TNFhi B cells were found to be negatively correlated with the number of involved lymph nodes. The frequency of TNFhi B cells showed an inverse correlation with the frequency of FoxP3+ Tregs, which in turn was associated with indicators of a poor prognosis.
Conclusions
Our data indicate that the cytokine profiles of B cells in TDLNs of patients with breast cancer show associations with various disease parameters. TNFhi and IL-10+ B cells correlated positively with indicators of a good prognosis. Further functional studies are required to elucidate the role of cytokine production by B cells in immunity against breast cancer.







Similar content being viewed by others
References
N.A. Giraldo, E. Becht, R. Remark, D. Damotte, C. Sautes-Fridman, W.H. Fridman, The immune contexture of primary and metastatic human tumours. Curr. Opin. Immunol. 27, 8–15 (2014)
T.L. Whiteside, Immune responses to cancer: Are they potential biomarkers of prognosis? Front. Oncol. 3, 107 (2013)
J. Mansfield, P. Lee, Immune-cancer interactions in tumors and tumor-draining lymph nodes: Novel prognostic indicators for breast cancer. J. Immunother. Cancer 2, 255 (2014)
Y. Vahidi, Z. Faghih, A.R. Talei, M. Doroudchi, A. Ghaderi, Memory CD4(+) T cell subsets in tumor draining lymph nodes of breast cancer patients: A focus on T stem cell memory cells. Cell Oncol. 41, 1–11 (2018)
N.J. Flynn, R. Somasundaram, K.M. Arnold, J. Sims-Mourtada, The multifaceted roles of B cells in solid tumors: Emerging treatment opportunities. Target. Oncol. 12, 139–152 (2017)
P. Tsou, H. Katayama, E.J. Ostrin, S.M. Hanash, The emerging role of B cells in tumor immunity. Cancer Res. 76, 5597–5601 (2016)
Q. Li, X. Lao, Q. Pan, N. Ning, J. Yet, Y. Xu, S. Li, A.E. Chang, Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin. Cancer Res. 17, 4987–4995 (2011)
J. Deng, J. Galipeau, B cells for cancer immunotherapy. OncoImmunology 3, e955702 (2014)
C.-A. Shin, H.-W. Cho, A.-R. Shin, H.-J. Sohn, H.-I. Cho, T.-G. Kim, Co-expression of CD40L with CD70 or OX40L increases B-cell viability and antitumor efficacy. Oncotarget 7, 46173 (2016)
J.Y. Shi, Q. Gao, Z.C. Wang, J. Zhou, X.Y. Wang, Z.H. Min, Y.H. Shi, G.M. Shi, Z.B. Ding, A.W. Ke, Z. Dai, S.J. Qiu, K. Song, J. Fan, Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin. Cancer Res. 19, 5994–6005 (2013)
M. Schwartz, Y. Zhang, J.D. Rosenblatt, B cell regulation of the anti-tumor response and role in carcinogenesis. J. Immunother. Cancer 4, 40 (2016)
S. Shalapour, J. Font-Burgada, G. Di Caro, Z. Zhong, E. Sanchez-Lopez, D. Dhar, G. Willimsky, M. Ammirante, A. Strasner, D.E. Hansel, C. Jamieson, C.J. Kane, T. Klatte, P. Birner, L. Kenner, M. Karin, Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98 (2015)
L. Qian, G.R. Bian, Y. Zhou, Y. Wang, J. Hu, X. Liu, Y. Xu, Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cent. Eur. J. Immunol. 40, 263–265 (2015)
W.W. Wang, X.L. Yuan, H. Chen, G.H. Xie, Y.H. Ma, Y.X. Zheng, Y.L. Zhou, L.S. Shen, CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget 6, 33486–33499 (2015)
X. Wei, Y. Jin, Y. Tian, H. Zhang, J. Wu, W. Lu, X. Lu, Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol. 37, 6581–6588 (2016)
P.B. Olkhanud, B. Damdinsuren, M. Bodogai, R.E. Gress, R. Sen, K. Wejksza, E. Malchinkhuu, R.P. Wersto, A. Biragyn, Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 71, 3505–3515 (2011)
M. Bodogai, C. Lee Chang, K. Wejksza, J. Lai, M. Merino, R.P. Wersto, R.E. Gress, A.C. Chan, C. Hesdorffer, A. Biragyn, Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 73, 2127–2138 (2013)
F. Mehdipour, M. Razmkhah, A. Hosseini, M. Bagheri, A. Safaei, A.R. Talei, A. Ghaderi, Increased B regulatory phenotype in non-metastatic lymph nodes of node-positive breast cancer patients. Scand. J. Immunol. 83, 195–202 (2016)
H.E. Kohrt, N. Nouri, K. Nowels, D. Johnson, S. Holmes, P.P. Lee, Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2, e284 (2005)
Z. Faghih, N. Erfani, M.R. Haghshenas, A. Safaei, A.R. Talei, A. Ghaderi, Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol. Lett. 158, 57–65 (2014)
F. Balkwill, Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371 (2009)
G.D. Kalliolias, L.B. Ivashkiv, TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016)
B.W. Tse, K.F. Scott, P.J. Russell, Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer 2012, 128965 (2012)
X. Chen, J.J. Oppenheim, Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 585, 3611–3618 (2011)
E.C. Lee, P. Zhan, R. Schallhom, K. Packman, M. Tenniswood, Antiandrogen-induced cell death in LNCaP human prostate cancer cells. Cell Death Differ. 10, 761–771 (2003)
L.F. Fajardo, H.H. Kwan, J. Kowalski, S.D. Prionas, A.C. Allison, Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 140, 539–544 (1992)
S.J. Leibovich, P.J. Polverini, H.M. Shepard, D.M. Wiseman, V. Shively, N. Nuseir, Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329, 630–632 (1987)
L. Schweigerer, B. Malerstein, D. Gospodarowicz, Tumor necrosis factor inhibits the proliferation of cultured capillary endothelial cells. Biochem. Biophys. Res. Commun. 143, 997–1004 (1987)
N.S. Zuckerman, H. Yu, D.L. Simons, N. Bhattacharya, V. Carcamo-Cavazos, N. Yan, F.M. Dirbas, D.L. Johnson, E.J. Schwartz, P.P. Lee, Altered local and systemic immune profiles underlie lymph node metastasis in breast cancer patients. Int. J. Cancer 132, 2537–2547 (2013)
F. Annunziato, L. Cosmi, F. Liotta, E. Lazzeri, R. Manetti, V. Vanini, P. Romagnani, E. Maggi, S. Romagnani, Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J. Exp. Med. 196, 379–387 (2002)
D. Mougiakakos, C.C. Johansson, R. Jitschin, M. Bottcher, R. Kiessling, Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood 117, 857–861 (2011)
X. Chen, X. Wu, Q. Zhou, O.M. Howard, M.G. Netea, J.J. Oppenheim, TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J. Immunol. 190, 1076–1084 (2013)
X. He, S. Landman, S.C. Bauland, J. van den Dolder, H.J. Koenen, I. Joosten, A TNFR2-agonist facilitates high purity expansion of human low purity Treg cells. PLoS One 11, e0156311 (2016)
M. Leclerc, S. Naserian, C. Pilon, A. Thiolat, G.H. Martin, C. Pouchy, C. Dominique, Y. Belkacemi, F. Charlotte, S. Maury, B.L. Salomon, J.L. Cohen, Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood 128, 1651–1659 (2016)
M. Nagar, J. Jacob-Hirsch, H. Vernitsky, Y. Berkun, S. Ben-Horin, N. Amariglio, I. Bank, Y. Kloog, G. Rechavi, I. Goldstein, TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J. Immunol. 184, 3570–3581 (2010)
X. Valencia, G. Stephens, R. Goldbach-Mansky, M. Wilson, E.M. Shevach, P.E. Lipsky, TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006)
Q. Zhang, F. Cui, L. Fang, J. Hong, B. Zheng, J.Z. Zhang, TNF-alpha impairs differentiation and function of TGF-beta-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J. Mol. Cell Biol. 5, 85–98 (2013)
X. Chen, Y. Nie, H. Xiao, Z. Bian, A.J. Scarzello, N.-Y. Song, A.L. Trivett, D. Yang, J.J. Oppenheim, TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci. Rep. 6, 32834 (2016)
H. Sepulveda, A. Cerwenka, T. Morgan, R.W. Dutton, CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J. Immunol. 163, 1133–1142 (1999)
L. Gorelik, Y. Bar-Dagan, M.B. Mokyr, Insight into the mechanism(s) through which TNF promotes the generation of T cell-mediated antitumor cytotoxicity by tumor bearer splenic cells. J. Immunol. 156, 4298–4308 (1996)
X. Chen, R. Hamano, J.J. Subleski, A.A. Hurwitz, O.M. Howard, J.J. Oppenheim, Expression of costimulatory TNFR2 induces resistance of CD4+FoxP3- conventional T cells to suppression by CD4+FoxP3+ regulatory T cells. J. Immunol. 185, 174–182 (2010)
A.P. Cope, M. Londei, N.R. Chu, S.B. Cohen, M.J. Elliott, F.M. Brennan, R.N. Maini, M. Feldmann, Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J. Clin. Invest. 94, 749–760 (1994)
R.M. Aspalter, M.M. Eibl, H.M. Wolf, Regulation of TCR-mediated T cell activation by TNF-RII. J. Leukoc. Biol. 74, 572–582 (2003)
J.M. Lykken, K.M. Candando, T.F. Tedder, Regulatory B10 cell development and function. Int. Immunol. 27, 471–477 (2015)
R. de Waal Malefyt, H. Yssel, J.E. de Vries, Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J. Immunol. 150, 4754–4765 (1993)
A.P. Vicari, C. Chiodoni, C. Vaure, S. Ait-Yahia, C. Dercamp, F. Matsos, O. Reynard, C. Taverne, P. Merle, M.P. Colombo, A. O'Garra, G. Trinchieri, C. Caux, Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 196, 541–549 (2002)
J. Emmerich, J.B. Mumm, I.H. Chan, D. LaFace, H. Truong, T. McClanahan, D.M. Gorman, M. Oft, IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72, 3570–3581 (2012)
K.L. Dennis, A. Saadalla, N.R. Blatner, S. Wang, V. Venkateswaran, F. Gounari, H. Cheroutre, C.T. Weaver, A. Roers, N.K. Egilmez, K. Khazaie, T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol. Res. 3, 806–814 (2015)
J.B. Mumm, J. Emmerich, X. Zhang, I. Chan, L. Wu, S. Mauze, S. Blaisdell, B. Basham, J. Dai, J. Grein, C. Sheppard, K. Hong, C. Cutler, S. Turner, D. LaFace, M. Kleinschek, M. Judo, G. Ayanoglu, J. Langowski, D. Gu, B. Paporello, E. Murphy, V. Sriram, S. Naravula, B. Desai, S. Medicherla, W. Seghezzi, T. McClanahan, S. Cannon-Carlson, A.M. Beebe, M. Oft, IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 20, 781–796 (2011)
H. Groux, F. Cottrez, M. Rouleau, S. Mauze, S. Antonenko, S. Hurst, T. McNeil, M. Bigler, M.G. Roncarolo, R.L. Coffman, A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162, 1723–1729 (1999)
I.H. Chan, V. Wu, S. McCauley, E.A. Grimm, J.B. Mumm, IL-10: Expanding the immune oncology horizon. Receptors Clin. Investig. 2 (2015)
I.H. Chan, V. Wu, M. Bilardello, B. Jorgenson, H. Bal, S. McCauley, P. Van Vlasselaer, J.B. Mumm, PEG-rIL-10 treatment decreases FoxP3(+) Tregs despite upregulation of intratumoral IDO. OncoImmunology 5, e1197458 (2016)
K.P. Papadopoulos, A. Naing, J.R. Infante, D.J. Wong, K.A. Autio, P.A. Ott, G.S. Falchook, M. Patel, S. Pant, D.W. Rasco. Anti-tumor activity of PEGylated human IL-10 (AM0010) in patients with pancreatic or colorectal cancer. Pain 1, 4.5 (2016)
Acknowledgements
This study was financially supported by grants from the Iran National Science Foundation (Grant No. 91004076), Shiraz Institute for Cancer Research (Grant No. ICR-100-508) and Shiraz University of Medical Sciences (Grant No. 91-6100). We thank K. Shashok (AuthorAID in the Eastern Mediterranean) for improving the text of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethical approval
This study was performed according to the ethical standards of the Ethical Committee of Shiraz University of Medical Sciences and in compliance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehdipour, F., Razmkhah, M., Faghih, Z. et al. The significance of cytokine-producing B cells in breast tumor-draining lymph nodes. Cell Oncol. 42, 381–395 (2019). https://doi.org/10.1007/s13402-019-00433-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-019-00433-3