Abstract
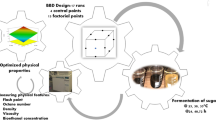
Bioethanol production from renewable biomass sources has garnered significant interest due to its potential as a sustainable alternative to fossil fuels. In this study, we investigated the optimization of bioethanol production from molasses, a by-product of the sugar production process using Saccharomyces cerevisiae through Response Surface Methodology (RSM). Initially, the fermentation process was optimized using RSM, considering four independent variables: substrate concentration, pH, temperature, and fermentation time. Subsequently, the effects of these variables on bioethanol yield were evaluated, and a quadratic model was developed to predict the optimum conditions. Analysis of variance (ANOVA) indicated a high coefficient of determination (R2) for the model, suggesting its adequacy for prediction. The optimized conditions for bioethanol production were determined as follows: substrate concentration of 200 g L−1, pH of 5.0, temperature of 30 °C and fermentation time of 72 h. Under these conditions, the predicted bioethanol yield was 84%. Overall, this study demonstrates the successful application of RSM for optimizing bioethanol production from molasses using S. cerevisiae, highlighting its potential as a promising feedstock for biofuel production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The increasing energy demands are predominantly met by fossil-based resources, presenting significant challenges [1]. Biofuels, often produced from agricultural products through various chemical methods, are utilized in mixture with gasoline and diesel as a clean energy alternative [2]. They are considered sustainable alongside solar and wind power, aiming to alleviate environmental and industrial concerns associated with future energy sources [3]. Liquid and gas-based biofuels, including bio-methanol, bioethanol, biobutanol, biomethane, biohydrogen, and biodiesel, have been extensively studied for their sustainability, economy, and usability [4]. The viability of these fuels depends on several technical factors, such as the sustainability of the fuel source, efficiency of conversion technologies, compatibility with existing engines, and engine performance [5]. The economic considerations encompass the cost per unit product consumed until the fuel reaches its final form, as well as the expense of necessary engine modifications. Economic considerations include the cost per unit product consumed during conversion and necessary engine modifications. Environmental factors also play a crucial role, influencing the suitability of a biofuel type, its potential to replace fossil resources, and the emissions resulting from combustion [6,7,8]. Among these biofuels, bioethanol stands out due to its versatility. Produced through processes like fermentation, it utilizes waste materials not suitable for agriculture. With its high-octane number and oxygen content enabling complete combustion, bioethanol improves the octane rating of gasoline while reducing hydrocarbon and CO emissions, thus mitigating the greenhouse effect. Additionally, bioethanol serves as a vital raw material in various chemical production processes [9, 10]. Bioethanol, derived through the fermentation process from substances or wastes rich in sugars such as glucose and sucrose, holds significant promise [11, 12]. Its versatility is highlighted by the fact that ethanol can be derived from various agricultural products and wastes, enabling domestic production in every country and reducing reliance on imports, thereby bolstering the global economy [13]. Recent advancements in bioethanol technologies, particularly its use as a fuel additive in gasoline blends, have led to the establishment of more efficient and productive ethanol production facilities [14]. Moreover, bioethanol production holds sectoral importance, as significant amounts of ethanol are utilized in the manufacture of disinfectants, detergents, and cosmetic products [15].
The process of ethanol production from plant sources varies depending on the available resources. For instance, when producing ethanol from lignocellulosic materials, a series of pretreatment and cellulose fragmentation processes are necessary. Conversely, if the production is from starchy materials, direct saccharification processes can be applied without pretreatments. Additionally, when ethanol is derived from raw materials like molasses, which contain free sugars, it can be directly subjected to fermentation [16, 17]. Regardless of the feedstock utilized, the production of ethanol requires a separation process post-fermentation to achieve the necessary purity levels. This separation process, typically conducted at temperatures ranging between 25 °C and 35 °C, usually spans between 36 and 72 h, depending on the type of yeast employed. The duration and efficiency of fermentation are influenced by factors such as sugar content and yeast activity. Therefore, optimizing efficiency under each experimental condition and with various raw materials is crucial in determining the most favorable conditions [18].
Recent advancements in bioethanol production technologies have significantly enhanced process efficiencies, facilitating the establishment of more productive ethanol production facilities. Optimization of fermentation processes, particularly utilizing non-conventional yeast strains like Meyerozyma caribbica MJTm3, has shown potential for enhancing bioethanol yields from feedstocks such as molasses, a by-product of the sugar industry [19]. A study conducted by Hawaz et al. (2022) underscores the potential of M. caribbica MJTm3, isolated from biowaste of sugar factories, to thrive under high ethanol concentrations, osmolarity, and temperature conditions, resulting in significant bioethanol concentrations from sugarcane molasses. The study employed Response Surface Methodology (RSM) based on a Central Composite Face-centered Design (CCFD) to optimize six fermentation parameters: temperature, pH, inoculum size, molasses concentration, mixing rate, and incubation period [20, 21]. These findings highlight the importance of harnessing advanced fermentation technologies and optimizing process parameters to improve bioethanol production efficiency.
This study optimizes bioethanol production from molasses using Saccharomyces cerevisiae and RSM, addresses technological challenges, and explores the potential of integrating bioethanol production into existing systems to enhance sustainability in the energy sector. By addressing technological challenges such as biomass pretreatment, enzymatic hydrolysis, and fermentation, this research aims to develop efficient, scalable, and sustainable bioethanol production processes from non-conventional feedstocks. Furthermore, the integration of bioethanol production with existing agricultural and energy systems is explored, aiming for a more sustainable and resilient energy future while reducing reliance on fossil fuels and mitigating climate change impacts. This study demonstrates the successful application of RSM in optimizing fermentation conditions, resulting in a high bioethanol yield of 84%, showcasing the potential of molasses as a promising biofuel feedstock.
2 Materials and methodology
2.1 Feedstock
Molasses is a type of sugar by-product that cannot crystallize and is generated through the refining process of sugar factories alongside the production of refined sugar [22]. Sugarcane molasses procured from Konya Sugar Factory, was stored at − 4 °C until utilized.
2.2 Media and preparation of yeast inoculum solution
To a solution containing sugarcane molasses at a concentration of 20% (w/v), 1% (w/v) of C6H5Na3O7.2H2O solution and 0.3% (w/v) NH4H2PO4 were added. The pH of the solution was adjusted to 4.0 using dilute sulfuric acid and sterilized by heating at 150 °C for approximately 10 min. Following cooling to room temperature, 1% (w/v) of Saccharomyces cerevisiae yeast was added. The solution was then placed in a shaking incubator at 30 °C for 18 h, the predetermined temperature for preparing the yeast inoculum solution. The molasses concentration and pH were adjusted based on experimental conditions, followed by sterilization at 150 °C for 15 min to prevent contamination. Subsequently, fermentation was initiated by adding 10% of the yeast inoculation solution prepared earlier.
2.3 Fermentation experiments and analytical methods
The fermentation experiments were conducted in 100 mL Erlenmeyer flasks equipped with a pipe from the outlet to a water-filled container, each containing 50 mL of culture media and inoculated with 10 mL of fresh yeast inoculum stock (approximately 10 mg of fresh yeast per mL). The process was carried out with magnetic stirrers set at 100 rpm, where the temperature was adjusted according to the specified experimental parameters. Samples for analysis were collected at the beginning and end of fermentation at designated periods. Process parameters are given in Table 1. At specific time intervals, 25 mL samples were taken from the solutions and underwent purification to isolate the ethanol content using a simple distillation unit. Following this procedure, the alcohol yields were quantified by analyzing the samples with a digital refractometer.
2.4 Experimental design and statistical analysis
The central composite face-centered design (CCFD) was employed to investigate process variables [23, 24]. The experimental studies were conducted according to a 24 full factorial design for the four identified design independent variables; these are fermentation duration (X1) (h), temperature (X2) (°C), initial pH value (X3), and molasses concentration (g/L) (X4), encompassing low (-1) and high (+ 1) levels. The design factors (variables) at low (-1) and high (+ 1) levels are, respectively, X1 [48 and 96 h], X2 [30 and 38 °C], X3 [4.5 and 5.5], and X4 [150 and 250 g/L]. The central values selected for experimental design; the zero level, respectively, are 72 h, 34 °C, 5.0, and 200 g/L (Tables 2 and 3). The statistical software Design Expert version 12 was utilized for designing experiments, conducting regression and graphical analyses of the obtained data, and performing statistical analysis on the model to assess the analysis of variance (ANOVA).
3 Results and discussion
3.1 Model equations
RSM, a widely-used mathematical and statistical technique, is employed to model and analyze processes affected by multiple variables, with the ultimate goal of optimizing the response [25, 26]. The parameters that influence the process are known as independent variables, while the responses are termed dependent variables. For example, ethanol efficiency is influenced by variables X1 (temperature) and X2 (pH), and its efficiency can vary across different combinations of X1 and X2. When determining independent variables, limit values were selected based on previous experimental findings [21]. In the RSM technique, experimental data are evaluated to fit a statistical model (Linear, Quadratic, Cubic, or 2FI (two-factor interaction)). Model coefficients include the constant term, A, B, and C (linear coefficients for independent variables), AB, AC, and BC (interaction term coefficients), and A2, B2, and C2 (quadratic term coefficients). Model adequacy is assessed using correlation coefficient (R2), adjusted determination coefficient (Adj-R2), and adequate precision; a model is deemed adequate if p-value < 0.05, lack of fit p-value > 0.05, R2 > 0.9, and Adeq. Precision > 4. Statistical significance between means is tested using analysis of variance (ANOVA). The suitable type of equation has been determined according to Tables 4 and 5. Accordingly, a quadratic model equation containing a second-degree polynomial was identified as suitable, as evidenced by the program’s output. Key factors contributing to this determination include the p-value and lack-of-fit p-value. Moreover, it’s vital to ensure agreement between the estimated and obtained regression coefficient (R2). As illustrated in the table, the model’s conformity to the experimental data is verified by a p-value below 0.05, a lack-of-fit p-value exceeding 0.05, and R2 values surpassing 0.8.
Additionally, ANOVA was conducted to assess the model’s suitability (Table 6). The F value, the most crucial parameter of ANOVA, is determined by the ratio of the mean model’s squared error to the residual error, with a higher F value indicating a more accurate model estimate. As shown in Table 6, the F value is 52.59, confirming the appropriateness of the surface response method. Significance is indicated when the error values of model data are < 0.05. Examining the error values of all model data, it was found that the p-values of A, B, D, BC, CD, A2, C2, and D2 were significant model terms as they were < 0.05, whereas the p-values of the other terms were deemed insignificant for the model as they were > 0.05. Table 7 presents the coefficients of the independent variables derived from the model, with those requiring consideration highlighted in bold. Besides, the fact that the Quadratic model produces an insignificant lack-of-fit confirms the suitability of the model and the lack-of-fit F value is 3.58, suggesting a 16.1% probability of such a large F-value occurring due to noise.
Another crucial factor of the model’s validity lies in its regression coefficients, with R2 values ideally surpassing 0.8. As illustrated in Table 8, the estimated R2 value slightly exceeds 0.92, the adjusted value is close to 0.96, and R2 itself is 0.98, affirming the model’s acceptability. The proximity between the predicted R2 value and the adjusted R2 value underscores their consistency when the difference between them is less than 0.2. Additionally, model adequacy is indicated by precision, where a value exceeding 4 is favorable. In this study, a precision value exceeding 24 signifies the model’s suitability within the designated design ranges and suggests minimal error when implemented.
Based on this data, the model equation for ethanol efficiency, the dependent variable reliant on temperature, pH, molasses concentration, and time, is formulated as follows:
Y = 78.42 + 1.3 A-6.78B-7.2D + 4.75BC-3.25CD-4.2A2 -8.7C2 -9.2D2.
3.2 Model accuracy graphs
Alongside the statistical data, comparison and diagnostic graphs were generated to validate the model. Residuals, representing the difference between model and experimental values, were utilized to assess the model’s fit. Figure 1 illustrates residual plots confirming the model’s fit, showing residuals closely aligned with a straight line, indicating model compatibility. Notably, in Fig. 1a and c, predicted and actual values exhibit minimal deviation from a linear trend, further validating the model’s accuracy. Besides, Fig. 1b displays the percentage probability plot against residuals externally. The points represent the experimental results and determine whether the acceptability falls within the range of ± 4 values.
3.3 3D and contour result graphs
3.3.1 The effect of pH
The pH value is a significant factor influencing bioethanol efficiency. Previous results indicates that ethanol production and efficiency decline when pH values fall below 4.0 or exceed 6.0, largely due to yeast activity [21, 27, 28]. Therefore, this study investigated pH effects within the range of 4.5 to 5.5. The impact of pH variation on molasses concentration, temperature, and pressure was assessed through graphical analysis (Fig. 2). While ethanol production typically rises with increasing molasses concentration under normal conditions, efficiency may not increase linearly. Hence, pH, temperature, and duration were individually evaluated for each molasses concentration. In Fig. 2, the combined influence of temperature and fermentation time is depicted at three pH values while maintaining molasses concentration at 200 g L−1. Notably, higher efficiency values were observed, particularly at pH 5.0, with efficiencies decreasing at pH 5.5. Similar results have been obtained in the literature [29]. An acidic environment promotes yeast activity, indicating that S.cerevisiae yeast exhibits acidophilic characteristics, leading to higher efficiencies under acidic conditions. Additionally, acidic pH facilitates the conversion of sucrose into fermentable sugars by invertase enzyme. Consequently, as pH rises and acidity diminishes, efficiencies decrease. In the pH graphs, the effects of time and temperature are depicted on the x and y axes. Particularly, it was noted that efficiency increases more noticeably as temperature decreases at low pH values, with the effect diminishing at higher pH values. Similarly, as pH increases, the impact of fermentation time decreases. However, the efficiency increment between 72 and 96 h is generally more significant than that between 48 and 72 h.
3.3.2 The effect of molasses concentration
Figure 3 depicts the impact of increasing molasses concentration on ethanol yield in an environment with a pH value of 5.0. As observed from the graphs, high efficiency values were achieved at molasses concentrations of 150 g L−1 and 200 g L−1. However, the efficiency decreased at a concentration of 250 g L−1. Although the ethanol yield expected at 250 g L−1 is higher, the actual yield decreases due to the constant amount of sugar that yeast can convert at consistent yeast concentrations. Similarly, despite low ethanol production at 150 g L−1, the yield remained high owing to the lower theoretical amount of ethanol. The reason for the decrease in yield when concentrations exceed 200 g L−1 is that yeast utilizes some sugars for growth, converting the remainder into alcohol. Hence, working with molasses concentrations between 150 g L−1 and 200 g L−1 is deemed more appropriate [30, 31]. Another observation from the graphs is that fermentation time slightly increases ethanol yield at low yeast concentrations, as ethanol is consistently transformed in the environment. Furthermore, Vučurović et al. observed that yeast cells encounter osmotic stress from high ethanol concentrations during molasses fermentation (initial sugar concentration above 250 g/L), leading to decreased metabolic activity, viability, and growth [32]. Similarly, another study reported significant osmotic stress in yeast cells under very high sugar concentration conditions (300 g/L sugar concentration), resulting in reduced growth and cell viability [33]. Consequently, a significant portion of fermentable sugars often remains unused due to inactivation during fermentation, highlighting the importance of initial molasses concentration.
3.3.3 The effect of temperature
Temperature is another important parameter that affects fermentation efficiency because various commercial yeasts and yeast strains react differently to temperature changes. Therefore, temperature profiles need to be integrated into product specifications or temperatures need to be tested before moving into large-scale production. Longer periods allow for greater sugar conversion, potentially maximizing ethanol yield but may lead to by-product accumulation. Conversely, shorter durations may limit fermentation efficiency. Balancing these factors is crucial for optimizing ethanol production while maintaining product quality. The fermentation temperature range was determined to be between 30 and 38 °C, and the relevant limits were entered into the program for optimization (Fig. 4) [34, 35]. The main reason for the decrease in ethanol efficiency due to temperature increase may be the inactivation of yeast at high temperatures. Here, the highest efficiency was obtained at 30 °C, which is consistent with previous studies [36, 37]. A sharp decrease in efficiency was observed after 34 °C. Consequently, fermentation processes must be planned around these temperature references. The graphs also show the effects of time and pH. The effect of pH follows a parabolic trend, initially increasing and then decreasing. The highest efficiencies were consistently achieved at pH 5. However, at lower temperatures, increasing pH to 5.5 slightly reduces efficiency, while at higher temperatures, increasing pH reduces efficiency by more than 20%. The effect of time is the same in all scenarios; no increase in productivity was observed after 72 h. In the previous studies, it has been observed that the temperature range of 30–32 °C is optimal for achieving maximum ethanol production [38]. This range likely facilitates the ideal metabolic activity of yeast cells, allowing for efficient fermentation of sugars into ethanol. Maintaining the fermentation temperature within this range can potentially enhance ethanol yields and overall process efficiency.
3.3.4 The effect of fermentation time
During fermentation, the substrate (sugar) and yeast interact in the environment from the early stages. However, yeasts, which utilize some time for reproduction, also produce ethanol during the remaining time [39]. Temperature significantly affects this production. Fermentation tends to finish faster at higher temperatures. However, ethanol production generally increases up to a certain time and then stabilizes if other conditions remain constant. Factors such as the presence of oxygen in the environment can convert ethanol into acidity, leading to decreased ethanol yield with increased time. Fermentation durations between 48 and 96 h have been observed in the literature, and optimization points were determined accordingly in the experiment [40]. According to previous studies, achieving high ethanol yield with optimal productivity in a batch fermentation system necessitates a longer fermentation period. This implies that shorter fermentation times and lower incubation temperatures lead to inefficient ethanol fermentation due to inadequate microbial growth. Moreover, overloading the fermentation vessel with substrate can result in a continuous fermentation rate, inducing osmotic shock in yeast cells, which has an inhibitory effect [41, 42]. The findings indicate that bioethanol production starts to decline immediately after 72 h of molasses concentration. Figure 5 illustrates the effect of different durations with 150 g L−1 of molasses usage. The influence of pH and temperature is also observed in the graphs. As seen in the figure, ethanol yield is low at 48 h, high at 72 and 96 h, and similar. However, it’s evident that holding for 96 h is unnecessary. Whether 72 h is sufficient or not could also be determined from the program. Accordingly, confirmation and point predictions found that 72–77 h is ideal. This comprehensive understanding of fermentation time optimization allows for efficient ethanol production while minimizing unnecessary time investments.
As a result, while the highest experimental ethanol efficiency in this study was calculated as 84%, it is possible to achieve an efficiency close to 86% under the optimization conditions outlined in Table 9. This demonstrates the potential for further improvement in efficiency through parameter optimization. Additionally, the validation chart in Table 10 emphasizes the highest response, ethanol efficiency, under varied independent variables, providing valuable insights into the robustness of the optimized conditions.
There are a few studies in the literature on the experimental design of ethanol production from molasses. El-Gendy et al. (2013) used CCD of RSM. In a batch fermentation procedure, they were able to produce a maximum of 255 g/L of ethanol at ideal operating conditions of roughly 71 h, pH 5.6, 38 °C, molasses concentration of 18% weight%, and 100 rpm. By examining the data from a 24 factorial design, a very significant (R2 = 9871, P < 0.0001) regression quadratic model equation was produced. The highest bioethanol yields that have been estimated and observed are 253 and 255 g/L, respectively [43]. Hawaz et al. (2024) used CCD of RSM for the optimization of molasses for bioethanol production. The following parameter was determined as ideal for highest ethanol yield: pH 5.5, 20% of the inoculum, 25% of the molasses concentration, at 30 C, 72 h of incubation. Analysis of variance was used to determine the quadratic model (ANOVA). F-test and p-values were used to regulate the statistical significance, and the model was determined to with R2 = 0.95. Therefore, it can be concluded that the bioethanol efficiency of 86% was comparable to the expected findings of 78.6% [44]. In a study conducted by Beigbeder et al. (2021), bioethanol production from sugar beet molasses was examined using the RSM, CCD technique. According to the data obtained, 82% ethanol efficiency was obtained under the conditions of minimum 0.4 g/L nutrient, 0.27 g/L yeast concentration and 150 g/L sugar solution to produce. In modeling, linear regression fit with an accuracy of 0.97 was obtained with p-values below 0.0006 and Lack of fit values 57.66 [45]. Homauda et al. (2025) also performed bioethanol production from molasses and used CCD of RSM model. According to the ANOVA analysis results fit with the quadratic model with an extremely low probability p-value of less than 0.0001, and a very high statistical significance at the 95% confidence level. The determination coefficients, 𝑅2 and 𝑅2 adj were found to be 0.953 and 0.920, respectively. The fermentation process reached its peak efficiency of 63.86% after 72 h [29]. In this study, quadratic model was fit with experimental values with R2 of 0.9827, p-values below 0.0001 and Lack of fit values 3.58. The highest efficiency of 84% was achieved at a molasses concentration of 200 g L−1, pH of 5, and temperature of 30 °C.
4 Conclusions
In conclusion, this study delved into the intricate dynamics of bioethanol production, meticulously examining the multifaceted variables influencing fermentation efficiency. Through a comprehensive analysis encompassing factors such as fermentation duration, temperature, pH, and molasses concentration, we elucidated the nuanced interplay shaping ethanol yield. The RSM has verified to be a reliable tool to identify key process variables and optimize bioethanol fermentation parameters using Saccharomyces cerevisiae from sugar beet processing by-product molasses. Additionally, the quadratic model and 3-D response surface plots were effective in predicting and exploring variations in bioethanol yield based on the experimental design. Through experimentation with molasses as the primary raw material, optimization efforts yielded promising results. The highest efficiency, reaching 84%, was achieved at a molasses concentration of 200 g L-1, pH of 5, and temperature of 30 °C. This study sheds light on the significance of molasses in alcohol production and provides insights into optimizing production processes for enhanced efficiency. Moving forward, the amalgamation of empirical research and computational modeling promises continued advancements in bioethanol production, paving the way for sustainable energy solutions in the future.
Data availability
Data will be made available on request.
References
Amadi PU, Ifeanacho MO (2016) Impact of changes in fermentation time, volume of yeast, and mass of plantain pseudo-stem substrate on the simultaneous saccharification and fermentation potentials of African land snail digestive juice and yeast. J Genet Eng Biotechnol 14:289–297. https://doi.org/10.1016/j.jgeb.2016.09.002
Kartini AM, Dhokhikah Y (2018) Bioethanol Production from Sugarcane molasses with simultaneous saccharification and fermentation (SSF) method using Saccaromyces Cerevisiae-Pichia stipitis Consortium. IOP Conf Ser Earth Environ Sci 207:012061. https://doi.org/10.1088/1755-1315/207/1/012061
Khan MAH, Bonifacio S, Clowes J, Foulds A, Holland R, Matthews JC, Percival CJ, Shallcross DE (2021) Investigation of Biofuel as a potential renewable energy source. Atm 12(10):1289. https://doi.org/10.3390/atmos12101289
Álvaro AG, Palomar CR, de Almeida Guimarães V, Hedo EB, Torre RM, de Godos Crespo I (2022) Developmental Perspectives of the Biofuel-Based Economy. In: Bandh SA, Malla FA (eds) Biofuels in Circular Economy. Springer, Singapore. https://doi.org/10.1007/978-981-19-5837-3_9
Kargbo H, Harris JS, Phan AN (2021) Drop-in fuel production from biomass: critical review on techno-economic feasibility and sustainability. Renew Sustain Energy Rev 135:110168. https://doi.org/10.1016/j.rser.2020.110168
Festel GW GW (2008) Biofuels – Economic aspects. Chem Eng Technol 31:715–720. https://doi.org/10.1002/ceat.200700335
Swana J, Yang Y, Behnam M, Thompson R (2011) An analysis of net energy production and feedstock availability for biobutanol and bioethanol. Bioresour Technol 102(2):2112–2117. https://doi.org/10.1016/j.biortech.2010.08.051
García V, Päkkilä J, Ojamo H, Muurinen E, Keiski RL (2011) Challenges in biobutanol production: how to improve the efficiency? Renew Sustain Energy Rev 15(2):964–980. https://doi.org/10.1016/j.rser.2010.11.008
Tse TJ, Wiens DJ, Reaney MJT (2021) Production of Bioethanol—A review of factors affecting ethanol yield. Ferment 7(4):268. https://doi.org/10.3390/fermentation7040268
Lamichhane G, Khadka S, Acharya A, Parajuli N (2023) Correction to: pretreatment of finger millet straw (Eleusine coracana) for enzymatic hydrolysis towards bioethanol production. Biomass Convers Biorefinery 13:7397–7397. https://doi.org/10.1007/s13399-021-01633-4
Dibazar AS, Aliasghar A, Behzadnezhad A, Shakiba A, Pazoki M (2023) Energy cycle assessment of bioethanol production from sugarcane bagasse by life cycle approach using the fermentation conversion process. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-04288-5
Duque A, Álvarez C, Doménech P, Manzanares P, Moreno AD (2021) Advanced Bioethanol production: from novel raw materials to Integrated Biorefineries. Processes 9(2):206. https://doi.org/10.3390/pr9020206
Valderrama C, Quintero V, Kafarov V (2020) Energy and water optimization of an integrated bioethanol production process from molasses and sugarcane bagasse: a Colombian case. Fuel 260:116314. https://doi.org/10.1016/j.fuel.2019.116314
Barua S, Sahu D, Sultana F, Baruah S, Mahapatra S (2023) Bioethanol, internal combustion engines and the development of zero-waste biorefineries: an approach towards sustainable motor spirit. RSC Sustainability 1:1065–1084. https://doi.org.10.1039/D3SU00080J
Stambuk BU (2014) Biotechnology strategies with industrial fuel ethanol Saccharomyces cerevisiae strains for efficient 1st and 2nd generation bioethanol production from sugarcane. BMC Proceedings 8:O36. https://doi.org/10.1186/1753-6561-8-S4-O36
Farahani SS, Asoodar MA (2017) Life cycle environmental impacts of bioethanol production from sugarcane molasses in Iran. Environ Sci Pollut Res 24:22547–22556. https://doi.org/10.1007/s11356-017-9909-1
Nigam PS, Singh A Production of liquid biofuels from renewable resources. Prog Energy Combust Sci. 37(1), 52–68. https://doi.org/10.1016/j.pecs.2010.01.003
Cardona CA, Sanchez OJ, Gutierrez LF (2020) Process Synthesis for Fuel Ethanol Production, 1st edn. CRC Press. https://doi.org/10.1201/9781439815984
Ahuja V, Arora A, Chauhan S, Thakur S, Jeyaseelan C, Paul D (2023) Yeast-mediated biomass valorization for Biofuel Production: A literature review. Ferment 9(9):784. https://doi.org/10.3390/fermentation9090784
Hawaz E, Tafesse M, Tesfaye A, Beyene D, Kiros S, Kebede G, Boekhout T, Theelen B, Groenewald M, Degefe A, Degu S, Admas A, Muleta D (2022) Isolation and characterization of bioethanol producing wild yeasts from bio-wastes and co-products of sugar factories. Ann Microbiol 72:39. https://doi.org/10.1186/s13213-022-01695-3
Hawaz E, Tafesse M, Tesfaye A, Kiros S, Beyene D, Kebede G, Boekhout T, Groenwald M, Theelen B, Degefe A, Degu S, Admasu A, Hunde B, Muleta D (2023) Optimization of bioethanol production from sugarcane molasses by the response surface methodology using Meyerozyma Caribbica isolate MJTm3. Ann Microbiol 73:2. https://doi.org/10.1186/s13213-022-01706-3
Altinisik S, Zeidan H, Yilmaz MD, Marti ME (2023) Reactive extraction of Betaine from Sugarbeet Processing byproducts. ACS Omega 8(12):11029–11038. https://doi.org/10.1021/acsomega.2c07845
Mostafa HS (2023) Potato peels for tannase production from Penicillium commune HS2, a high tannin-tolerant strain, and its optimization using response surface methodology. Biomass Convers Biorefinery 13:16779–16779. https://doi.org/10.1007/s13399-021-02205-2
Veljković VB, Veličković AV, Avramović JM, Stamenković OS (2019) Modeling of biodiesel production: performance comparison of Box–Behnken, face central composite and full factorial design. Chin J Chem Eng 27(7):1690–1698. https://doi.org/10.1016/j.cjche.2018.08.002
Khuri AI, Mukhopadhyay S (2010) Response surface methodology. WIRE Comput Stat 2:128–149. https://doi.org/10.1002/wics.73
Alev Yüksel A (2018) Utilization of response surface methodology in optimization of extraction of plant materials, in: S. Valter (Ed.). Statistical approaches with emphasis on design of experiments applied to chemical processes. IntechOpen, Rijeka, p Ch 10. https://doi.org/10.5772/intechopen.73690
Narendranath NV, Power R (2005) Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl Environ Microbiol 71(5):2239–2243. https://doi.org/10.1128/AEM.71.5.2239-2243.2005
Raby HS, Saadat MA, Sakib AN, Moni Chowdhury F, Yousuf A (2023) Bioethanol production from sugarcane molasses with supplemented nutrients by industrial yeast. Biofuels 15(2):129–135. https://doi.org/10.1080/17597269.2023.2221880
Hamouda HI, Nassar HN, Madian HR, Abu Amr SS, El-Gendy NS (2015). Response surface optimization of bioethanol production from sugarcane molasses by Pichia veronae strain HSC‐22. Biotech Res Int 2015(1):905792. https://doi.org/10.1155/2015/905792
Cazetta ML, Celligoi MAPC, Buzato JB, Scarmino IS (2007) Fermentation of molasses by Zymomonas mobilis: effects of temperature and sugar concentration on ethanol production. Bioresour Technol 98(15):2824–2828. https://doi.org/10.1016/j.biortech.2006.08.026
Nguyen HP, Du Le H, Man Le VV (2015) Effect of ethanol stress on Fermentation performance of Saccharomyces cerevisiae cells immobilized on Nypa fruticans Leaf Sheath Pieces. Food Technol Biotechnol 53(1):96–101. https://doi.org/10.17113/ftb.53.01.15.3617
Vučurović VM, Puškaš VS, Miljić UD (2018) Bioethanol production from sugar beet molasses and thick juice by free and immobilised Saccharomyces cerevisiae. J Brew Sci 125(1):134–142. https://doi.org/10.1002/jib.536
Razmovski R, Vučurović V (2012) Bioethanol production from sugar beet molasses and thick juice using Saccharomyces cerevisiae immobilized on maize stem ground tissue. Fuel 92(1):1–8. https://doi.org/10.1016/j.fuel.2011.07.046
Darvishi F, Abolhasan Moghaddami N (2019) Optimization of an Industrial Medium from molasses for Bioethanol Production using the Taguchi Statistical Experimental-Design Method. Ferment 5(1):14. https://doi.org/10.3390/fermentation5010014
Muruaga ML, Carvalho KG, Domínguez JM, de Souza Oliveira RP, Perotti N (2016) Isolation and characterization of Saccharomyces species for bioethanol production from sugarcane molasses: studies of scale up in bioreactor. Renewable Energy 85:649–656. https://doi.org/10.1016/j.renene.2015.07.008
Huang J, Wang X, Chen X, Li H, Chen Y, Hu Z, Yang S (2023) Adaptive Laboratory Evolution and Metabolic Engineering of Zymomonas mobilis for Bioethanol Production using molasses. ACS Synth Biol 12(4):1297–1307. https://doi.org/10.1021/acssynbio.3c00056
Saithong P, Nakamura T, Shima J (2009) Prevention of bacterial contamination using acetate-tolerant Schizosaccharomyces Pombe during bioethanol production from molasses. J Biosci Bioeng 108(3):216–219. https://doi.org/10.1016/j.jbiosc.2009.03.022
Sanchez N, Cobo M, Rodriguez-Fontalvo D, Uribe-Laverde MÁ, Ruiz-Pardo RY (2021) Bioethanol Production from Sugarcane Press-Mud: Assessment of the Fermentation Conditions to Reduce Fusel Alcohol. Fermentation 7(3):194. https://doi.org/10.3390/fermentation7030194
da Silva Fernandes F, de Souza ÉS, Carneiro LM, Alves Silva JP, de Souza JVB, da Silva Batista J (2022) Current ethanol production requirements for the yeast saccharomyces cerevisiae. Int J Microbiol. 2022:7878830. https://doi.org/10.1155/2022/7878830
Gutiérrez-Rivera B, Ortiz-Muñiz B, Gómez-Rodríguez J, Cárdenas-Cágal A, Domínguez González JM, Aguilar-Uscanga MG (2015) Bioethanol production from hydrolyzed sugarcane bagasse supplemented with molasses B in a mixed yeast culture. Renewable Energy 74:399–405. https://doi.org/10.1016/j.renene.2014.08.030
Cavalaglio G, Gelosia M, Ingles D, Pompili E, D’Antonio S, Cotana F (2016) Response surface methodology for the optimization of cellulosic ethanol production from Phragmites australis through pre-saccharification and simultaneous saccharification and fermentation. Ind Crops Prod 83:431–437. https://doi.org/10.1016/j.indcrop.2015.12.089
Azhar SHM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM, Rodrigues KF (2017) Yeasts in sustainable bioethanol production: A review. Biochemistry and Biophysics Reports 10:52–61. https://doi.org/10.1016/j.bbrep.2017.03.003
El-Gendy NS, Madian HR, Amr SSA (2013) Design and optimization of a process for sugarcane molasses fermentation by Saccharomyces cerevisiae using response surface methodology. Int J Microbio 2013. https://doi.org/10.1155/2013/815631
Hawaz E, Tafesse M, Tesfaye A, Kiros S, Beyene D, Kebede G, Boekhout T, Groenewald M, Theelen B, Degefe A, Degu S, Admasu A, Hunde B, Muleta D (2023) Optimization of bioethanol production from sugarcane molasses by the response surface methodology using Meyerozyma Caribbica isolate MJTm3. Ann Microbiol 73(1):2. https://doi.org/10.21203/rs.3.rs-2196564/v1
Beigbeder JB, de Medeiros Dantas JM, Lavoie JM (2021) Optimization of yeast, sugar and nutrient concentrations for high ethanol production rate using industrial sugar beet molasses and response surface methodology. Fermentation 7(2):86. https://doi.org/10.3390/fermentation7020086
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by the Office of Scientific Research Projects Coordination at Çanakkale Onsekiz Mart University. Grant number: FIA-2020-3312.
Author information
Authors and Affiliations
Contributions
Sinem Altınışık: conceptualization, investigation, writing-review and editing. Filiz Uğur Nigiz: conceptualization, investigation, methodology. Savaş Gürdal: investigation, writing-original draft. Kadir Yılmaz: investigation, writing-original draft. Necati Barış Tuncel: investigation, methodology. Sermet Koyuncu: conceptualization, funding acquisition, writing-review and editing.
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altınışık, S., Nigiz, F.U., Gürdal, S. et al. Optimization of bioethanol production from sugar beet processing by-product molasses using response surface methodology. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05786-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05786-w