Abstract
This study aimed to investigate the biosorption performance of acid-modified waste Prunus mahaleb (PMA) shells in the removal of Pb2+ ions from aqueous solutions. Changes in the morphological properties and functional components of PMA biosorbent were characterized using SEM–EDX, FT-IR, BET, and PZC analyses. The effect of various parameters such as initial Pb2+ concentration, pH, PMA dosage, contact time, and temperature on biosorption was investigated using a batch biosorption procedure. The maximum biosorption capacity, determined using the Langmuir isotherm, was calculated to be 119 mg g−1. It was found that the biosorption kinetic mechanism followed pseudo-second-order kinetics and intraparticle diffusion model. According to the determined thermodynamic parameters, the biosorption mechanism was found to be endothermic (ΔH° > 0), spontaneous (ΔS° > 0), and entropy-increasing (ΔG° < 0). The outcomes of the experiment were evaluated in comparison to other sorbents that have been previously commonly used in the literature. It was demonstrated that PMA could be a promising, environmentally friendly, cost-effective, and sustainable potential biosorbent for the removal of Pb2+ ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Two of the most significant challenges facing humanity on a global scale are access to clean water and water pollution. In recent years, the rate of heavy metals and synthetic chemicals in clean water and wastewater has been increasing as a result of anthropogenic activities and rapid industrialization [1]. This increase in water pollution is attributed to the uncontrolled release of wastewater filled with heavy metals and organic pollutants from sectors such as battery and nuclear production, metal processing, mining, agriculture, dyeing, pesticide and fertilizer industry, and chemical and pharmaceutical industries [2]. Heavy metals such as lead, zinc, arsenic, mercury, and cobalt, which can be found in dissolved or particle form, pose significant risks to ecological life due to their persistence, non-biodegradability, and stability in the environment [3]. Heavy metals exhibit their toxicity at low concentrations of approximately 1.0–10.0 mg L−1 [4]. If the human body is exposed to these heavy metals, health problems such as cancer; miscarriages in pregnant women; brain, liver, kidney, and bone damage; nervous system damage; and neurological disorders may occur [5].
Lead is one of the most well-known toxic heavy metal pollutants. It poses significant risks to both the environment and human health due to its toxic properties and persistence in batteries and paints [6]. Lead exists in two valences: Pb2+ and Pb4+. However, Pb2+, which is the most common in the oxidation state, is more toxic to living things due to its higher bioavailability and mobility [7]. As a result of exposure to Pb2+ metal above specified limits and its accumulation in living systems, problems such as hypertension, hematological and reproductive problems, and kidney and brain damage may occur. In addition, Pb2+, which can be found in low concentrations in wastewater, can also cause anemia and hepatitis. The World Health Organization has reported that permissible levels of Pb in drinking water may be 0.01 mg L−1 [8, 9].
Studies are being carried out to develop many physical, chemical, and biological systems that will be useful in removing various impurities from aqueous solutions. Techniques such as flocculation, coagulation, ultrafiltration, nanofiltration, membrane separation, and electrochemical separation can be given as examples [10, 11]. However, these techniques have negative aspects such as expensive initial installation and operating costs, secondary pollution resulting from the process, and low removal capacity [12]. Among these techniques, adsorption is preferred by many researchers because it is highly selective, eco-friendly, simple, and low-cost, and because of its regeneration ability.
In addition, the efficiency of adsorption varies depending on the surface area width and morphology of the preferred adsorbent, pore size and volume, polarity, and functional compounds required for adsorption [13]. A wide variety of adsorbents, especially activated carbon, have been evaluated for the removal of heavy metals. Due to the high production cost of activated carbon, which is very effective in heavy metal removal, research has recently been carried out for easily obtainable, sustainable, and cost-effective adsorbents with high adsorption capacity [14]. This research contributes to the broader goal of developing environmentally friendly and economically feasible water treatment solutions. The utilization of natural materials and waste products from industrial and agricultural activities as low-cost alternative adsorbents is a promising approach in the field of heavy metal ion removal. Choosing agricultural wastes as adsorbents serves an environmental purpose and also saves on the high preparation costs of adsorbents. This strategy aligns with the principles of sustainability, cost-effectiveness, and eco-friendly waste utilization. Agricultural wastes are a category of lignocellulosic biomass consisting of cellulose, hemicellulose, and lignin. In addition, most of these wastes contain many functional groups such as carboxyl, amino, aldehyde, and phenolic groups. It increases the efficiency of agricultural waste in heavy metal removal due to the presence of these functional groups with metal binding properties and porous surface properties [15].
However, various physical and chemical modifications need to be applied to agricultural-based adsorbents to increase their adsorption performance, structural stability, and reusability. Physical modification includes radiation, application of mechanical force, and thermal treatments with gases such as carbon dioxide and nitrogen, which improve the surface structure of adsorbents. Chemical modification enables the inclusion of certain functional groups into adsorbents by undergoing various processes with chemical substances (HCl, H2SO4, KOH, NaOH, etc.) [16,17,18,19,20,21]. With these modifications, the adsorption capacity is improved by increasing the surface porosity of the adsorbents.
Different lignocellulosic agricultural wastes, whose adsorption properties were improved with various chemical reagents, have been investigated as adsorbents in Pb2+ removal in many studies [12, 22,23,24].
Prunus mahaleb (PM), a member of the Rosaceae family, is a member of the St. It is known as Lucie cherry, rock cherry, or mahaleb cherry [25]. PM, which can easily grow in the Mediterranean, Central Europe, and Western Asia, is used in the pharmaceutical industry due to its cardioprotective, immune system strengthening, antidiabetic, diuretic, and expectorant effects, and in the food industry due to its aromatic taste. Export of raw materials or processed products is extremely important economically for Türkiye [26, 27].
A review of the literature reveals that the biosorption potential of a multitude of biomass and agricultural wastes has been investigated as biosorbents for the removal of heavy metals. However, there is no data on Pb2+ removal by acid-modified Prunus mahaleb shells (PMA).
Therefore, in this study, PM shells, which are abundant agricultural wastes without commercial value, were modified with HCl and it was aimed to determine the biosorption capacity in Pb2+ removal from aqueous solution for the first time.
Physicochemical properties of PMA biosorbent were determined by FT-IR, SEM–EDX, BET, and zero charge point (pHPZC) analyses. The effects of different values of parameters such as solution pH, initial metal concentration, amount of adsorbent, contact time, and temperature on the biosorption process were examined.
2 Method
2.1 Materials
The Pb(NO3)2 and 4-(2-pyridyl azo) resorcinol (PAR), which were employed to investigate the biosorption of Pb2+ ions onto PMA, were procured from Merck. The following chemicals were purchased from Sigma-Aldrich: potassium nitrate (KNO3), hydrochloric acid (HCl), sodium hydroxide (NaOH), ethanol (C2H5OH), and methanol (CH3OH). All chemicals were of analytical purity. All experiments were conducted in duplicate using double-distilled water.
2.2 Preparation of biosorbent
PM shells were obtained as waste from a factory exporting mahaleb in Tokat province. To remove physical impurities, the shells were washed with distilled water several times and then dried in an oven at 65 °C for 48 h. To increase the surface area, the shells were ground to smaller sizes using a grinder (IKA A 11). The prepared shells were stored in propylene airtight containers until further processing.
2.3 Modification of biosorbent
PM shells, cleared of impurities, were shaken with 1 M HCl at a ratio of 3:1 for 24 h. Acid-modified PM shells (PMA) were treated with double-distilled water until neutral to remove excess acid not covalently bound to the biosorbent surface.
The obtained PMA biosorbent (Fig. 1) was then dried in an oven to remove any remaining moisture. Once dried, the biosorbent was stored in airtight tubes until it was used in experiments.
2.4 Biosorption experiments
Biosorption experiments to examine the removal of Pb2+ ions from aqueous solutions were carried out by batch method by adding 100 mg PMA to 1000 mg L−1 and 10 mL Pb2+ solutions at pH 4.5 for 24 h at an ambient temperature 25 °C. The metal complex formed by PAR of Pb2+ ions remaining in the equilibrium solution after biosorption was determined by measuring at λ = 518 nm using the UV–vis spectrophotometric method [28].
To prepare the stock Pb2+ solution with a concentration of 1000 mg L−1, 1.598 g Pb(NO3)2 was dissolved in distilled water in a 1-L volumetric bottle. The Pb2+ solutions with a concentration range of 50–1000 mg L−1 were obtained by diluting the stock Pb2+ solution with distilled water. These solutions were employed to investigate the effect of initial Pb2+ concentration on the biosorption process. The effect of contact time was determined by running 2–1440 min in 30 mL solutions containing 300 mg PMA at a fixed Pb2+ concentration of 1000 mg L−1.
While different amounts of PMA of 10, 30, 50, 100, and 200 mg were used to determine the effect of biosorbent dose, constant Pb2+ concentration was studied at different temperatures such as 5, 25, and 40 °C to investigate the effect of temperature. To examine the effect of ambient pH value on biosorption, the pH values of Pb2+ solutions were adjusted between 1 and5 with dilute 0.1 M HCl and 0.1 M NaOH.
The amount of Pb2+ adsorbed by the unit mass of the adsorbent during the equilibrium period was calculated with Eq. (1), the Pb2+ biosorption percentage was calculated with Eq. (2), and Recovery% were calculated using Eq. (3) [29].
where Co (mg L−1) represents the initial Pb2+ solution concentration, Ce (mg L−1) represents the Pb2+ solution concentration in the equilibrium period, m (g) represents the amount of adsorbent, and V (L) represents the solution volume. Qdes is desorbed amount of Pb2+ (mg g−1), and Qads is the adsorbed amount of Pb2+ (mg g−1).
2.5 Determination of the point of zero charge (pHPZC)
To ascertain the variation in the surface charge of the biosorbent by the ambient pH during the biosorption process, the solid addition method was employed to determine the zero point charge (pHPZC). A total of 100 mg of PMA was added to 10 mL of 0.1 M KNO3 solutions with pH values ranging from 2 to 12. After 24 h, the final pH (pHf) of the equilibrium solutions was measured. The pHPZC value was determined as the point where the line obtained from plotting pHi-ΔpH (pHi-pHf) intersected the X-axis [30].
3 Results and dıscussıon
3.1 FT-IR analysis
FT-IR spectra recorded for PMA biosorbent before and after Pb2+ biosorption are shown in Fig. 2. With FT-IR, functional groups of PMA before biosorption and changes in the densities of these groups after Pb2+ biosorption were detected.
The broad and intense peak centered at approximately 3333 cm−1 observed in the FT-IR spectrum of PMA before biosorption in Fig. 2 consists of O–H stretching vibrations of alcohol, carboxylic, and phenolic compounds in the high cellulose and lignin content of PMA [31]. It can be thought that the peaks determined in the 2925 and 2858 cm−1 spectra are caused by the symmetric and asymmetric C-H stretching of the methyl and methylene groups of lignocellulosic structures [32].
The peak determined at 1732 cm−1 is due to the stretching vibration of the C = O bond of hemicellulose [25], while the peak determined at 1689 and 1637 cm−1 is caused by the aromatic C = O stretching vibration in the carbonyl group in lignin.
The peak at 1511 cm−1 is due to C–C stretching vibrations of the aromatic ring in lignin [33].
The strong peak observed at 1028 cm−1 can be attributed to the symmetric or asymmetric vibration of the C–O–C, C-O, and C–OH groups of cellulose [34].
Upon examination of the FT-IR spectrum of PMA following Pb2+ biosorption, a notable decrease in the intensities of all characteristic absorption peaks was observed. A notable reduction in the intensity of the broad and broadened peak attributed to the O–H stretching vibration was observed, while it was determined that one of the peaks corresponding to the C-H stretching shifted to 2908 cm−1 and the other disappeared. The observed decreases and changes can be attributed to the electrostatic interactions between the functional groups and Pb2+ ions, as well as the surface complexation reactions [35].
3.2 SEM and EDX analyses
EDX-assisted SEM analysis was performed to examine the morphological properties and elemental changes of PMA before and after Pb2+ biosorption. SEM images of PMA biosorbent before and after biosorption are shown in Fig. 3. In Fig. 3a, it can be seen that the acid-modified shells are quite porous and these pores are open. In Fig. 3b, it was determined that after Pb2+ biosorption, the pores were filled and layers were formed on the surface. These results determined by SEM images were confirmed by EDX spectra obtained for PMA before and after interaction with Pb2+ ions (Fig. 3d). It was observed that the C and O peaks seen in the EDX spectrum before biosorption decreased significantly after Pb2+ biosorption. It was also determined that Pb and N peaks appeared after biosorption. These observed changes in the EDX spectrum were also determined in other studies investigating Pb2+ biosorption [36].
Therefore, both the morphological changes observed in SEM images and the elemental changes in the EDX spectra are considered evidence supporting the conclusion that Pb2+ ions were successfully biosorbed by the PMA material.
3.3 BET-porosity analysis
BET surface area (m2/g) analysis was performed for raw PM and PMA. While the BET surface area for PM was determined as 0.9737 m2 g−1, the BET surface area for PMA was determined to increase to 5.71 m2 g−1. Therefore, it appears that the modification process affects the outer surface of the stone. Based on these data, it is thought that modification with HCl increases the number of active binding sites to hold more metal and, as a result, improves its biosortive properties.
3.4 Effect of pH on biosorption
Solution pH represents a crucial variable that influences the efficiency of adsorption by modifying the surface characteristics of the adsorbent and the interactions between the adsorbent and the adsorbate [37]. This can be attributed to the strong competition between H+ ions and metal ions. To investigate the influence of the initial solution pH on the biosorption of Pb2+ ions onto PMA, a series of experiments were conducted utilizing 1000 mg L−1 Pb2+ solutions with pH levels spanning from 1.0 to 5.0. The outcomes of these experiments are presented in Fig. 4.
Nevertheless, some studies in the literature indicate that the biosorbent may become unusable as Pb2+ ions precipitate as Pb(OH)2 above pH 5.5, resulting in the formation of toxic sludge and secondary pollution [38]. Consequently, examining it at higher pH levels was not feasible. Figure 4 illustrates that an increase in biosorption efficiency and capacity can be observed as the pH value rises [39].
Pb2+ removal using PMA was determined to be most effective at pH 4.0. However, the biosorption efficiency began to decline after this pH. At low pH values, the competition between H+ ions and Pb2+ ions to bind to active sites on the biosorbent surface results in a low biosorption amount.
As the pH value increases, the number of protons in the solution decreases, which results in a reduction in competition between Pb2+ ions and active binding sites. This enhanced accessibility of active binding sites results in an augmented biosorption quantity of Pb2+ ions. However, the observed decrease in biosorption efficiency and capacity between pH 4 and 5 is attributed to the formation of soluble hydroxylated complexes of Pb2+ ions, which compete with active sites for binding.
Furthermore, the pH value at which the electrical charge of the PMA surface is neutral (pHPZC) was determined to be 2.61. This indicates that the surface charge of the PMA biosorbent is positive at pH values below 2.61 and negative at pH values above 2.61. When the surface charge of PMA is positive, there are electrostatic repulsion forces between Pb2+ ions, which results in a reduction in biosorption efficiency. Conversely, when the surface charge of PMA is negative, there are electrostatic attraction forces between Pb2+ ions, resulting in an increase in biosorption efficiency [40, 41].
3.5 Effect of biosorbent dose on biosorption
The dosage of preferred biosorbent is an important parameter that determines the efficiency and cost of biosorption. Biosorption of Pb2+ ions with different PMA amounts (1–20 g L−1) was investigated by keeping other experimental parameters constant. Based on the experimental data obtained, the biosorption% of Pb2+ ions and the relationship between the biosorption capacity of PMA and the amount of biosorbent are shown in Fig. 5. As can be seen from Fig. 5, as the amount of PMA increased in batch biosorption studies, the percentage biosorption of Pb2+ ions also increased. Maximum biosorption of 84.9% was determined with 20 g L−1 PMA biosorbent. This increase can be attributed to the increase in active binding sites and surface area in direct proportion to the increase in the amount of biosorbent. Thus, more Pb2+ ions will adhere to the PMA surface.
This increase can be attributed to the increase in active binding sites and surface area in direct proportion to the increase in the amount of PMA. Thus, more Pb2+ ions will adhere to the PMA surface. However, in Fig. 5, a decrease in biosorption capacity was observed as the amount of PMA increased. The reason for this decrease can be shown as the overlap of active binding sites as the amount of PMA increases, the diffusion path length increases, and the sites are not sufficiently saturated with Pb2+ [42]. The effects of PMA amount on biosorption% and biosorption capacity observed in this study have been reported with similar results as studies in the literature [43].
3.6 Biosorption isotherms
Three isotherm models, Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) models, were used to study biosorption isotherms. The Langmuir isotherm is a model that explains monolayer adsorption occurring in homogeneous regions on the adsorbent surface and the balance between the adsorbent and the adsorbate. The Freundlich isotherm is used to describe multilayer adsorption occurring in heterogeneous regions on the adsorbent surface. The D-R isotherm model is used to determine the activation energy and physical and chemical nature of adsorption on a non-homogeneous surface with Gaussian energy distribution [44, 45].
The equations and calculated parameters of these isotherm models are given in Table 1. Biosorption isotherms of Pb2+ ions by PMA biomass are shown in Fig. 6.
When examining the correlation coefficient (R2) values presented in Table 1, it is evident that both the Langmuir and Freundlich models fit well with the experimental data; this suggests the coexistence of monolayer and multilayer adsorption behavior [46].
From the Langmuir model, the maximum biosorption capacity of the monolayer was calculated as 119 mg g−1 and the Langmuir equilibrium constant, which represents the affinity of the binding sites, was calculated as 0.00238 L mg−1. The Freundlich constant, representing the biosorption capacity, was determined as 1.06 L mg−1, and the β value, representing the biosorption intensity, was determined as 0.670. The presence of β < 1 indicates the formation of new active sites for biosorption and an increase in biosorption capacity. The determined β indicates the suitability of the biosorption process.
The average free energy of Pb2+ ions (EDR) on PMA was calculated as 8.04 kJ mol−1 using the D-R model. The obtained EDR value, which is greater than 8 kJ mol−1, indicates that the biosorption process is chemical in nature. Furthermore, it was demonstrated that the correlation coefficient (R2 = 0.983) obtained for the D-R model is consistent with the equilibrium data.
A comparison of the sorption capacities of various adsorbents used in Pb2+ biosorption is given in Table 2. As a result, it is seen that the Pb2+ ion biosorption performance of PMA is higher than of other sorbents.
3.7 Biosorption kinetics
Adsorption kinetics is an important property describing the effectiveness of adsorption, which refers to the rate at which adsorbates are retained or released at the solid–liquid interface. Therefore, to better understand the kinetics of Pb2+ biosorption on PMA biosorbent, pseudo-first-order (PFO) [55], pseudo-second-order (PSO) [56], and intraparticle diffusion (IPD) [30] kinetic models were applied. The equations of these kinetic models and the calculated kinetic parameters are presented in Table 3. The fit of the kinetic models applied for the biosorption of Pb2+ ions onto PMA biomass is seen in Fig. 7.
As seen in Fig. 7, it was determined that Pb2+ ions adhered to PMA biomass quickly for up to 180 min and reached equilibrium within 240 min. The high initial Pb2+ removal can be attributed to the presence of sufficient active sites for adsorption. However, as the contact time increases, Pb2+ removal will reach equilibrium when the active sites for biosorption decrease [57].
When the correlation coefficients (R2) obtained from the graph were evaluated, it was determined that the R2 = 0.925 value of the PSO kinetic model was greater than the R2 = 0.899 value of the PFO kinetic model (Table 3).
Moreover, the proximity of the experimentally calculated Qe,exp, and the calculated Qe,cal values using the equations of the PSO model provided further evidence of the compatibility of the PSO model.
In line with the data obtained, it can be concluded that biosorption kinetics occurs through the PSO kinetic model.
The PSO model describes the chemical adsorption process in which electrostatic interaction and hydrogen bonds occur between the adsorbent and the adsorbate. Moreover, according to this kinetic model, the efficiency of biosorption is directly proportional to the available active sites [58]. In addition, to explain in more detail the step that controls the diffusion rate in Pb2+ biosorption onto PMA biomass, all experimental data were examined in more detail by applying the linear form of the IPD model. The IPD model includes linear regions with different slopes and helps explain the stages of the biosorption process [59]. As seen in Fig. 7, according to the IPD model, the biosorption of Pb2+ ions onto PMA biomass consists of a two-step mechanism. The first slope shows the rapid adhesion of Pb2+ from the aqueous solution to the biosorbent surface, and the second slope shows the diffusion of Pb2+ ions into the biosorbent pores until they reach equilibrium [60].
3.8 Biosorption thermodynamics
Thermodynamic studies are critical to determine the effect of temperature on the biosorption process. Studies were carried out at temperatures of 5 °C, 25 °C, and 40 °C to explain the thermodynamic behaviors of the biosorption of Pb2+ ions onto PMA biomass. The thermodynamic parameters of enthalpy change (∆H°), entropy (∆S°), and Gibbs free energy (∆G°) were calculated. These parameters are given in Table 4. The isothermal change between ∆H° and ∆S° parameters was determined by Eq. (4), called the Van’t Hoff equation [61].
where R represents the ideal gas constant (8.314 J mol−1 K−1), T (K) represents the absolute experimental temperature, and KD represents the distribution coefficient. KD distribution coefficient is calculated with Eq. (5).
According to the Van’t Hoff equation, ∆H° and ∆S° parameters were calculated with the help of the slope and intercept of the line obtained from the lnKD − 1/T graph in Fig. 8. ∆G° is calculated using Eq. (6), which is also related to ΔH° and ΔS° at constant temperature.
Determining the adsorption enthalpy value as a positive value (ΔH° = 13.2 kJ mol−1) indicates that the Pb2+ removal process occurring on the PMA biosorbent surface is endothermic. Additionally, biosorption entropy was calculated as 76.4 J mol−1 K−1. Positive determination of ΔH° and ΔS° values indicated that the affinity between Pb2+ ions and PMA biosorbent is sufficient for biosorption and the randomness between the solid–liquid interface increases [62]. As seen in Table 4, negative ΔG° values indicate that biosorption is spontaneous, while negative values increase with increasing temperature, indicating that the efficiency of biosorption increases at higher temperatures. Similar results have been reported in other studies in the literature [35, 36].
4 Recovery
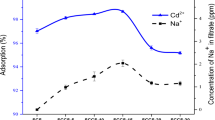
After the biosorption process, the effective reuse of the biosorbent is a critical factor affecting the cost of the biosorption process. Additionally, it is important in terms of being feasible, sustainable, and not creating secondary pollution. Therefore, in the study, recovery studies of Pb2+ ions from PMA were conducted, utilizing 0.1 M HCl, ethanol, and methanol solutions. The adsorption/desorption cycle was repeated three times using the same biosorbent. The concentration of Pb2+ remaining in the equilibrium solutions following each process was determined by the spectrophotometric method. The results of the study are presented in Fig. 9. Figure 9 illustrates that the ethanol solution (62%) is more effective than the HCl (24%) and methanol (28%) solutions in the Pb2+ desorption process. However, when the recovery percentage is examined, it can be concluded that the biosorbed Pb2+ ions diffuse into the particle or are chemically bound [63]. In light of these data, PMA can be considered a promising biosorbent for the recovery of Pb2+ ions.
5 Conclusion
The objective of this study was to investigate the biosorption properties of chemically modified PMA biosorbent (prepared using 1 M HCl) for the removal of Pb2+ ions. FT-IR, SEM–EDX, and BET analyses were conducted and the resulting data was interpreted in order to determine the structural properties of PMA. The pHPZC value of PMA was calculated to be 2.61. Biosorption isotherm studies of Pb2+ ions were conducted by applying the Langmuir, Freundlich, and D-R isotherm models. The correlation coefficients obtained were found to be similar, indicating that biosorption was compatible with both the Langmuir and Freundlich isotherm models. The maximum biosorption capacity of the monolayer, as determined by the Langmuir isotherm model, was calculated to be 119 mg g−1. The average free energy (EDR, 8.04 kJ mol−1) obtained by applying the D-R isotherm model indicated that the biosorption process proceeded chemically. The application of experimental data to PFO, PSO, and IPD kinetic models revealed that biosorption reached equilibrium in 240 min at 25 °C. Furthermore, the biosorption kinetic mechanism was found to be compatible with the PSO and IPD models. Thermodynamic studies have demonstrated that Pb2+ removal is endothermic (ΔH° > 0) and spontaneous (ΔG° < 0). Furthermore, it was established that the affinity between Pb2 + ions and functional groups on the biosorbent surface was conducive to biosorption (ΔS° > 0). In recovery experiments conducted with HCl, ethanol, and methanol to investigate the reusability of PMA, it was determined that the highest efficiency was obtained with ethanol. The data obtained from this study indicate that PMA, a chemically modified agricultural waste, could be a low-cost, abundant, potential, and effective biosorbent that does not create secondary pollution in removing Pb2+ ions.
Data availability
Not applicable.
References
An HK, Park BY, Kim DS (2001) Crab shell for the removal of heavy metals from aqueous solution. Water Res 35:3551–3556. https://doi.org/10.1016/S0043-1354(01)00099-9
Burakov AE, Galunin EV, Burakova IV et al (2018) Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review. Ecotoxicol Environ Saf 148:702–712. https://doi.org/10.1016/J.ECOENV.2017.11.034
El-Gaayda J, Titchou FE, Oukhrib R et al (2021) Natural flocculants for the treatment of wastewaters containing dyes or heavy metals: a state-of-the-art review. J Environ Chem Eng 9:106060. https://doi.org/10.1016/j.jece.2021.106060
Chakraborty R, Asthana A, Singh AK et al (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Int J Environ Anal Chem 102:342–379. https://doi.org/10.1080/03067319.2020.1722811
Khan Rao RA, Khatoon A (2017) Aluminate treated Casuarina equisetifolia leaves as potential adsorbent for sequestering Cu(II), Pb(II) and Ni(II) from aqueous solution. J Clean Prod 165:1280–1295. https://doi.org/10.1016/j.jclepro.2017.07.160
Al-Homaidan AA, Al-Abbad AF, Al-Hazzani AA et al (2016) Lead removal by Spirulina platensis biomass. Int J Phytoremediation 18:184–189. https://doi.org/10.1080/15226514.2015.1073673
Ludolphy C, Kierdorf U, Kierdorf H (2021) Lead concentrations in antlers of European roe deer (Capreolus capreolus) from an agricultural area in Northern Germany over a 119-year period—a historical biomonitoring study. Environ Sci Pollut Res 28:56069–56078. https://doi.org/10.1007/s11356-021-14538-6
Iqbal M, Khera RA (2015) Adsorption of copper and lead in single and binary metal system onto Fumaria indica biomass. Chem Int 1:157b–163b
Lalhruaitluanga H, Jayaram K, Prasad MNV, Kumar KK (2010) Lead(II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)-a comparative study. J Hazard Mater 175:311–318. https://doi.org/10.1016/j.jhazmat.2009.10.005
Mustapha S, Shuaib DT, Ndamitso MM et al (2019) Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl Water Sci 9:1–11. https://doi.org/10.1007/s13201-019-1021-x
Saravanan A, Kumar PS, Yaashikaa PR et al (2021) Mixed biosorbent of agro waste and bacterial biomass for the separation of Pb(II) ions from water system. Chemosphere 277:130236. https://doi.org/10.1016/j.chemosphere.2021.130236
Syeda HI, Sultan I, Razavi KS, Yap PS (2022) Biosorption of heavy metals from aqueous solution by various chemically modified agricultural wastes: a review. J Water Process Eng 46:102446. https://doi.org/10.1016/j.jwpe.2021.102446
Ali RM, Hamad HA, Hussein MM, Malash GF (2016) Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol Eng 91:317–332. https://doi.org/10.1016/J.ECOLENG.2016.03.015
Singh S, Kumar V, Datta S et al (2020) Current advancement and future prospect of biosorbents for bioremediation. Sci Total Environ 709:135895. https://doi.org/10.1016/J.SCITOTENV.2019.135895
Jain CK, Malik DS, Yadav AK (2016) Applicability of plant based biosorbents in the removal of heavy metals: a review. Environ Process 3:495–523. https://doi.org/10.1007/s40710-016-0143-5
Kane SN, Mishra A, Dutta AK (2016) International Conference on Recent Trends in Physics (ICRTP 2016). J Phys Conf Ser 755:011001. https://doi.org/10.1088/1742-6596/755/1/011001
Dauda B, Abugu H, Alum OL (2020) Chromium adsorption using modified locust bean and maize husk chromium adsorption using modified locust bean and maize husk. Der Pharma Chem 12:7–14
Abugu H, Okoye P (2015) Preparation and characterization of activated carbon produced from oil bean (Ugba or Ukpaka) and snail shell. J Environ Anal Chem 02:1–19. https://doi.org/10.4172/2380-2391.1000165
Onyeka H, Pac O, Ajiwe VI, Ofordile P (2014) Preparation and characterisation of activated carbon from agrowastes peanut seed ( African Canarium ) and palm kernel shell. Int J Innov Res Dev 3:418
Abugu HO, Alum OL, Ihedioha JN et al (2023) Sequestration of Pb2+ from aqueous solution using bio-based-alkaline modified sorbent from waste Irvingia gabonensis seed husk. Water Pract Technol 18:2495–2513. https://doi.org/10.2166/wpt.2023.170
Simić M, Petrović J, Šoštarić T et al (2022) A mechanism assessment and differences of cadmium adsorption on raw and alkali-modified agricultural waste marija. Processes 10:1–13. https://doi.org/10.3390/pr10101957
Ahmadi H, Hafiz SS, Sharifi H et al (2022) Low cost biosorbent (melon peel) for effective removal of Cu (II), Cd (II), and Pb (II) ions from aqueous solution. Case Stud Chem Environ Eng 6:100242. https://doi.org/10.1016/j.cscee.2022.100242
Mahmood-ul-Hassan M, Suthar V, Rafique E et al (2015) Kinetics of cadmium, chromium, and lead sorption onto chemically modified sugarcane bagasse and wheat straw. Environ Monit Assess 187:4692. https://doi.org/10.1007/s10661-015-4692-2
Nuhoğlu Y, Ekmekyapar Kul Z, Kul S et al (2021) Pb (II) biosorption from the aqueous solutions by raw and modified tea factory waste (TFW). Int J Environ Sci Technol 18:2975–2986. https://doi.org/10.1007/s13762-020-03038-8
Ercisli S, Orhan E (2008) Fatty acid composition of seeds of yellow, red, and black colored Prunus mahaleb fruits in Turkey. Chem Nat Compd 44:87–89. https://doi.org/10.1007/s10600-008-0024-x
Özçelik B, Koca U, Kaya DA, Şekeroǧlu N (2012) Evaluation of the in vitro bioactivities of mahaleb cherry (Prunus mahaleb L.). Rom Biotechnol Lett 17:7863–7872
Bayrakçeken Güven Z, Dogan Z, Saracoglu I et al (2022) Food plant with antioxidant, tyrosinase inhibitory and antimelanoma activity: Prunus mahaleb L. Food Biosci 48:101804. https://doi.org/10.1016/j.fbio.2022.101804
Keskin ZS (2023) Efficient adsorption of Pb(II) ions using novel adsorbent polyacrylamide/coffee ground composite: isotherm, kinetic and thermodynamic studies. Polym Bull. https://doi.org/10.1007/s00289-023-05111-x
Şenol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isothems, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648. https://doi.org/10.1016/j.ijbiomac.2021.05.130
Şenol ZM, Keskin ZS, Şimşek S (2023) Synthesis and characterization of a new hybrid polymer composite (pollene@polyacrylamide) and its applicability in uranyl ions adsorption. J Radioanal Nucl Chem 332:2239–2248. https://doi.org/10.1007/s10967-023-08820-9
Taşar Ş, Kaya F, Özer A (2014) Biosorption of lead(II) ions from aqueous solution by peanut shells: equilibrium, thermodynamic and kinetic studies. J Environ Chem Eng 2:1018–1026. https://doi.org/10.1016/j.jece.2014.03.015
Kostić M, Radović M, Mitrović J et al (2014) Using xanthated Lagenaria vulgaris shell biosorbent for removal of Pb(II) ions from wastewater. J Iran Chem Soc 11:565–578. https://doi.org/10.1007/s13738-013-0326-1
Shi J, Li J (2012) Metabolites and chemical group changes in the wood-forming tissue of Pinus koraiensis under inclined conditions. BioResources 7:3463–3475. https://doi.org/10.15376/biores.7.3.3463-3475
Keskin ZS, Mine Şenol Z, Kaya S, Şimşek S (2023) Prunus mahaleb shell as a sustainable bioresource for carminic acid removal from aqueous solution: experimental and theoretical studies. J Mol Struct 1275:134618. https://doi.org/10.1016/j.molstruc.2022.134618
Petrović M, Šoštarić T, Stojanović M et al (2017) Mechanism of adsorption of Cu2+ and Zn2+ on the corn silk (Zea mays L.). Ecol Eng 99:83–90. https://doi.org/10.1016/j.ecoleng.2016.11.057
Petrović M, Šoštarić T, Stojanović M et al (2016) Removal of Pb2+ ions by raw corn silk (Zea mays L.) as a novel biosorbent. J Taiwan Inst Chem Eng 58:407–416. https://doi.org/10.1016/j.jtice.2015.06.025
Boddu S, Chandra A, Ali Khan A (2022) Biosorption of Cu(II), Pb(II) from electroplating industry effluents by treated shrimp shell. Mater Today Proc 57:1520–1527. https://doi.org/10.1016/j.matpr.2021.12.052
Şenol ZM, Şimşek S (2020) Equilibrium, kinetics and thermodynamics of Pb(II) ions from aqueous solution by adsorption onto chitosan-dolomite composite beads. Int J Environ Anal Chem 102:4926–4940. https://doi.org/10.1080/03067319.2020.1790546
Abugu HO, Eze SI, Ezugwu AL et al (2023) Chemical pretreatment of Lagenaria breviflora seeds used as biosorbents for the removal of aqueous-bound Ni2þ. Water Pract Technol 18:2514–2535. https://doi.org/10.2166/wpt.2023.192
Fatombi JK, Lartiges B, Aminou T et al (2013) A natural coagulant protein from copra (Cocos nucifera): isolation, characterization, and potential for water purification. Sep Purif Technol 116:35–40. https://doi.org/10.1016/j.seppur.2013.05.015
Reddy DHK, Harinath Y, Seshaiah K, Reddy AVR (2010) Biosorption of Pb(II) from aqueous solutions using chemically modified Moringa oleifera tree leaves. Chem Eng J 162:626–634. https://doi.org/10.1016/j.cej.2010.06.010
Pandey R, Ghazi Ansari N, Lakhan Prasad R, Chandra Murthy R (2014) Pb(II) Removal from aqueous solution by Cucumis sativus (cucumber) peel: kinetic, equilibrium & thermodynamic study. Am J Environ Prot 2:51–58. https://doi.org/10.12691/env-2-3-1
Petrović MS, Šoštarić TD, Pezo LL et al (2015) Usefulness of ann-based model for copper removal from aqueous solutions using agro industrıal waste materials. Chem Ind Chem Eng Q 21:249–259. https://doi.org/10.2298/CICEQ140510023P
Al-Mur BA (2023) Green zinc oxide (ZnO) nanoparticle synthesis using mangrove leaf extract from Avicenna marina: properties and application for the removal of toxic metal ions (Cd2+ and Pb2+). Water (Switzerland) 15:1–26. https://doi.org/10.3390/w15030455
Singh K, Singh AK, Kumar A, Agarwal A (2023) Fly ash and TiO2 modified fly ash as adsorbing materials for removal of Cd(II) and Pb(II) from aqueous solutions. J Hazard Mater Adv 10:100256. https://doi.org/10.1016/j.hazadv.2023.100256
Manfrin J, Gonçalves Junior AC, Schwantes D et al (2021) Effective Cd2+ removal from water using novel micro-mesoporous activated carbons obtained from tobacco: CCD approach, optimization, kinetic, and isotherm studies. J Environ Heal Sci Eng 19:1851–1874. https://doi.org/10.1007/s40201-021-00740-8
Batool F, Mohyuddin A, Amjad A et al (2023) Removal of Cd(II) and Pb(II) from synthetic wastewater using Rosa damascena waste as a biosorbent: an insight into adsorption mechanisms, kinetics, and thermodynamic studies. Chem Eng Sci 280:119072. https://doi.org/10.1016/j.ces.2023.119072
Tee WT, Loh NYL, Hiew BYZ et al (2022) Effective remediation of lead(II) wastewater by Parkia speciosa pod biosorption: Box-Behnken design optimisation and adsorption performance evaluation. Biochem Eng J 187:108629. https://doi.org/10.1016/j.bej.2022.108629
Thompson CO, Ndukwe AO, Asadu CO (2020) Application of activated biomass waste as an adsorbent for the removal of lead (II) ion from wastewater. Emerg Contam 6:259–267. https://doi.org/10.1016/j.emcon.2020.07.003
Lavado-Meza C, De la Cruz-Cerrón L, Cisneros-Santos G et al (2023) Arabica-coffee and teobroma-cocoa agro-industrial waste biosorbents, for Pb(II) removal in aqueous solutions. Environ Sci Pollut Res 30:2991–3001. https://doi.org/10.1007/s11356-022-22233-3
Ezeonuegbu BA, Machido DA, Whong CMZ et al (2021) Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol Reports 30:e00614. https://doi.org/10.1016/j.btre.2021.e00614
Das A, Bar N, Das SK (2020) Pb(II) adsorption from aqueous solution by nutshells, green adsorbent: adsorption studies, regeneration studies, scale-up design, its effect on biological indicator and MLR modeling. J Colloid Interface Sci 580:245–255. https://doi.org/10.1016/j.jcis.2020.07.017
Ezugwu AL, Abugu HO, Ucheana IA et al (2023) Sequestration of lead ion in aqueous solution onto chemically pretreated Pycnanthus angolensis seed husk: implications for wastewater treatment. Sustainability 15:15446. https://doi.org/10.3390/su152115446
Eze SI, Abugu HO, Odewole OA et al (2022) Thermal and chemical pretreatment of Terminalia mantaly seed husk biosorbent to enhance the adsorption capacity for Pb2+. Sci African 15:e01123. https://doi.org/10.1016/j.sciaf.2022.e01123
Vareda JP (2023) On validity, physical meaning, mechanism insights and regression of adsorption kinetic models. J Mol Liq 376:121416. https://doi.org/10.1016/j.molliq.2023.121416
Tran HN (2023) Applying linear forms of pseudo-second-order kinetic model for feasibly identifying errors in the initial periods of time-dependent adsorption datasets. Water (Switzerland) 15:1231. https://doi.org/10.3390/w15061231
Zhang SQ, Hou WG (2008) Adsorption behavior of Pb(II) on montmorillonite. Colloids Surfaces A Physicochem Eng Asp 320:92–97. https://doi.org/10.1016/j.colsurfa.2008.01.038
Boonchuay A, Worathanakul P (2022) The diffusion behavior of CO2 adsorption from a CO2/N2 gas mixture on zeolite 5A in a fixed-bed column. Atmosphere (Basel) 13:1–15. https://doi.org/10.3390/atmos13040513
Mohammadzadeh F, Golshan M, Haddadi-Asl V, Salami-Kalajahi M (2023) Adsorption kinetics of methylene blue from wastewater using pH-sensitive starch-based hydrogels. Sci Rep 13:1–15. https://doi.org/10.1038/s41598-023-39241-z
Naga Jyothi MSV, Harafan A, Sen Gupta S et al (2022) Chitosan immobilised granular FeOOH-MnxOybimetal-oxides nanocomposite for the adsorptive removal of lead from water. J Environ Chem Eng 10:107353. https://doi.org/10.1016/j.jece.2022.107353
Abdul Rahim AR, Mohsin HM, Thanabalan M et al (2020) Effective carbonaceous desiccated coconut waste adsorbent for application of heavy metal uptakes by adsorption: equilibrium, kinetic and thermodynamics analysis. Biomass Bioenerg 142:105805. https://doi.org/10.1016/j.biombioe.2020.105805
Zhu L, Yao Y, Chen D, Lan P (2022) The effective removal of Pb2+ by activated carbon fibers modified by l-cysteine: exploration of kinetics, thermodynamics and mechanism. RSC Adv 12:20062–20073. https://doi.org/10.1039/d2ra01521h
Şenol ZM, Şimşek S, Özer A, Şenol Arslan D (2021) Synthesis and characterization of chitosan–vermiculite composite beads for removal of uranyl ions: isotherm, kinetics and thermodynamics studies. J Radioanal Nucl Chem 327:159–173. https://doi.org/10.1007/s10967-020-07481-2
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The present study was supported by the Sivas Cumhuriyet University Projects Commission.
Author information
Authors and Affiliations
Contributions
Zehra Saba Keskin: experimental work, visualization, methodology, supervision, writing, guidance, editing, and review. Zeynep Mine Şenol: experimental work, visualization, methodology writing, editing, and review. Selçuk Şimşek: editing, and review.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keskin, Z.S., Şenol, Z.M. & Şimşek, S. The valorization of Prunus mahaleb shell through acid modification for the sorption of Pb2+ removal from aqueous solution. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05775-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05775-z