Abstract
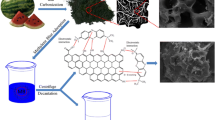
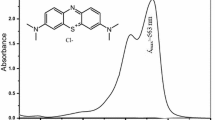
Acacia nilotica was used for the adsorption of Reactive Black 5 (RB5) dye from an aqueous solution. Both the raw and activated (with H3PO4) carbon forms of Acacia nilotica (RAN and ANAC, respectively) were used for comparison. Various parameters (including dye concentration, contact time, temperature, and pH) were optimized to obtain the maximum adsorption capacity. RAN and ANAC were characterized using Fourier transform infrared spectroscopy, scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The maximum experimental adsorption capacities for RAN and ANAC were 34.79 and 41.01 mg g−1, respectively, which agreed with the maximum adsorption capacities predicted by the Langmuir, Freundlich, and Dubinin–Radushkevich equilibrium isotherm models. The adsorption data of ANAC showed a good fit to the isotherm models based on the coefficient of determination (R 2): Langmuir type II (R 2 = 0.99) > Freundlich (R 2 = 0.9853) > Dubinin–Radushkevich (R 2 = 0.9659). This result suggested monolayer adsorption of RB5 dye. The adsorption of RB5 dye followed pseudo-second-order kinetics. The RAN adsorbent reflected an exothermic reaction (enthalpy change, ΔH = −0.006 kJ mol−1) and increased randomness (standard entropy change, ΔS = 0.038 kJ mol−1) at the solid–solution interface. In contrast, ANAC reflected both exothermic [−0.011 kJ mol−1 (303–313 K)] and endothermic [0.003 kJ mol−1 (313–323 K)] reactions. However, the ΔS value of ANAC was lower when the RB5 adsorption increased from 313 to 323 K. The negative values for the Gibbs free energy change at all temperatures indicated that the adsorption of RB5 dye onto RAN and ANAC was spontaneous in the forward direction.









Similar content being viewed by others
References
Ahmad AA, Hameed BH (2010) Fixed-bed adsorption of reactive azo dye onto granular activated carbon prepared from waste. J Hazard Mater 175:298–303. doi:10.1016/j.jhazmat.2009.10.003
Aksu Z, Akın AB (2010) Comparison of Remazol Black B biosorptive properties of live and treated activated sludge. Chem Eng J 165:184–193. doi:10.1016/j.cej.2010.09.014
Argun ME, Güclü D, Karatas M (2014) Adsorption of Reactive Blue 114 dye by using a new adsorbent: Pomelo peel. J Ind Eng Chem 20:1079–1084. doi:10.1016/j.jiec.2013.06.045
Ashiq MN, Najam-Ul-Haq M, Amanat T et al (2012) Removal of methylene blue from aqueous solution using acid/base treated rice husk as an adsorbent. Desalination Water Treat 49:376–383. doi:10.1080/19443994.2012.719467
Bulgariu D, Bulgariu L (2012) Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour Technol 103:489–493. doi:10.1016/j.biortech.2011.10.016
Chakraborty S, De S, DasGupta S, Basu JK (2005) Adsorption study for the removal of a basic dye: experimental and modeling. Chemosphere 58:1079–1086. doi:10.1016/j.chemosphere.2004.09.066
Daneshvar E, Kousha M, Sohrabi MS et al (2012) Biosorption of three acid dyes by the brown macroalga Stoechospermum marginatum: isotherm, kinetic and thermodynamic studies. Chem Eng J 195–196:297–306. doi:10.1016/j.cej.2012.04.074
Diao Y, Walawender WP, Fan LT (2002) Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresour Technol 81:45–52. doi:10.1016/S0960-8524(01)00100-6
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. doi:10.1016/j.cej.2009.09.013
Garg SK, Tripathi M, Lal N (2015) Response surface methodology for optimization of process variable for reactive orange 4 dye discoloration by Pseudomonas putida SKG-1 strain and bioreactor trial for its possible use in large-scale bioremediation. Desalination Water Treat 54:3122–3133. doi:10.1080/19443994.2014.905975
Girgis BS, El-Hendawy A-NA (2002) Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater 52:105–117. doi:10.1016/S1387-1811(01)00481-4
Gupta VK, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90. doi:10.1016/j.cej.2011.11.006
Hameed BH (2009) Removal of cationic dye from aqueous solution using jackfruit peel as non-conventional low-cost adsorbent. J Hazard Mater 162:344–350. doi:10.1016/j.jhazmat.2008.05.045
Hamzeh Y, Ashori A, Azadeh E, Abdulkhani A (2012) Removal of Acid Orange 7 and Remazol Black 5 reactive dyes from aqueous solutions using a novel biosorbent. Mater Sci Eng C Mater Biol Appl 32:1394–1400. doi:10.1016/j.msec.2012.04.015
Ho Y-S, McKay J, Wase DAJ, Forster CF (2000) Study of the sorption of divalent metal ions on to peat. Adsorpt Sci Technol 18:639–650. doi:10.1260/0263617001493693
Huang C-C, Li H-S, Chen C-H (2008) Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J Hazard Mater 159:523–527. doi:10.1016/j.jhazmat.2008.02.051
Jagtoyen M, Derbyshire F (1998) Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 36:1085–1097. doi:10.1016/S0008-6223(98)00082-7
Joseph CG, Ashri WM, Daud WMAW, Sanmugam K (2015) Parametric and adsorption kinetic studies of Reactive Black 5 removal from textile simulated wastewater using oil palm (Elais guineensis) empty fruit bunch. J Appl Sci 15:1103–1111. doi:10.3923/jas.2015.1103.1111
Kilislioglu A, Bilgin B (2003) Thermodynamic and kinetic investigations of uranium adsorption on amberlite IR-118H resin. Appl Radiat Isot Data Instrum Methods Use Agric Ind Med 58:155–160
Min Y, Meng Z, Bin L et al (2013) Characteristics of amine surfactant modified peanut shell and its sorption property for Cr(VI). Chin J Chem Eng 21:1260–1268. doi:10.1016/S1004-9541(13)60621-7
Moradi O (2016) Applicability comparison of different models for ammonium ion adsorption by multi-walled carbon nanotube. Arab J Chem 9(Supplement 2):S1170–S1176. doi:10.1016/j.arabjc.2011.12.014
Nabil GM, El-Mallah NM, Mahmoud ME (2014) Enhanced decolorization of Reactive Black 5 dye by active carbon sorbent-immobilized-cationic surfactant (AC–CS). J Ind Eng Chem 20:994–1002. doi:10.1016/j.jiec.2013.06.034
O’Mahony T, Guibal E, Tobin JM (2002) Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb Technol 31:456–463. doi:10.1016/S0141-0229(02)00110-2
Osma JF, Saravia V, Toca-Herrera JL, Couto SR (2007) Sunflower seed shells: a novel and effective low-cost adsorbent for the removal of the diazo dye Reactive Black 5 from aqueous solutions. J Hazard Mater 147:900–905. doi:10.1016/j.jhazmat.2007.01.112
Ozdes D, Gundogdu A, Kemer B et al (2014) Assessment of kinetics, thermodynamics and equilibrium parameters of Cr(VI) biosorption onto Pinus brutia Ten. Can J Chem Eng 92:139–147. doi:10.1002/cjce.21820
Padmesh TVN, Vijayaraghavan K, Sekaran G, Velan M (2006) Biosorption of Acid Blue 15 using fresh water macroalga Azolla filiculoides: batch and column studies. Dyes Pigments 71:77–82. doi:10.1016/j.dyepig.2005.06.003
Pirbazari AE, Saberikhah E, Badrouh M, Emami MS (2014) Alkali treated Foumanat tea waste as an efficient adsorbent for methylene blue adsorption from aqueous solution. Water Resour Ind 6:64–80. doi:10.1016/j.wri.2014.07.003
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255. doi:10.1016/S0960-8524(00)00080-8
Saba B, Jabeen M, Khalid A et al (2015) Effectiveness of rice agricultural waste, microbes and wetland plants in the removal of Reactive Black-5 azo dye in microcosm constructed wetlands. Int J Phytoremediation 17:1060–1067. doi:10.1080/15226514.2014.1003787
Sajab MS, Chia CH, Zakaria S, Khiew PS (2013) Cationic and anionic modifications of oil palm empty fruit bunch fibers for the removal of dyes from aqueous solutions. Bioresour Technol 128:571–577. doi:10.1016/j.biortech.2012.11.010
Sakkayawong N, Thiravetyan P, Nakbanpote W (2005) Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J Colloid Interface Sci 286:36–42. doi:10.1016/j.jcis.2005.01.020
Suárez-García F, Martinez-Alonso A, Tascón JMD (2002) Pyrolysis of apple pulp: chemical activation with phosphoric acid. J Anal Appl Pyrolysis 63:283–301. doi:10.1016/S0165-2370(01)00160-7
Vijayaraghavan K, Yun Y-S (2008) Biosorption of C.I. Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed Laminaria sp. Dyes Pigments 76:726–732. doi:10.1016/j.dyepig.2007.01.013
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AIChE J 20:228–238. doi:10.1002/aic.690200204
Yang Y-I, Jung D-W, Bai D-G et al (2001) Counterion-dye staining method for DNA in agarose gels using crystal violet and methyl orange. Electrophoresis 22:855–859. doi:10.1002/1522-2683()22:5<855:AID-ELPS855>3.0.CO;2-Y
Zhu H-Y, Yao J, Jiang R et al (2014) Enhanced decolorization of azo dye solution by cadmium sulfide/multi-walled carbon nanotubes/polymer composite in combination with hydrogen peroxide under simulated solar light irradiation. Ceram Int 40:3769–3777. doi:10.1016/j.ceramint.2013.09.043
Acknowledgements
The project was financially supported by King Saud University, Vice Deanship of Research Chairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Amin, M.T., Alazba, A.A. Comparative study of the absorptive potential of raw and activated carbon Acacia nilotica for Reactive Black 5 dye. Environ Earth Sci 76, 581 (2017). https://doi.org/10.1007/s12665-017-6927-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6927-8