Abstract
Schinus molle seeds and leaves are frequently employed in traditional medicine to treat a range of diseases. This study aims to explore the potential of Schinus mole (L.) seed and leaf extracts in terms of their phytochemical constituents and antimicrobial, antioxidant, and cytotoxic activities. The study utilized GC-MS spectroscopy, antimicrobials, antioxidants, and cytotoxicity techniques to evaluate the extracts. The result of phytochemical analysis revealed that Schinus molle seed extract contains seven peaks with a major compound, bis (2-ethylhexyl) phthalate (59.11%), followed by n-hexadecanoic acid (10.84), while Schinus molle leaf extract exhibits sixteen peaks with a main compound, squalene (16.87%), followed by azulene (14.88%) and lupeol (12.4%). The Schinus molle seeds and leaf exhibited the high antimicrobial activity with inhibition zones ranging from 15 ± 0.57 to 27.33 ± 0.88 mm against tested microorganisms. In addition, it was investigated that the minimum inhibitory concentrations (MICs) of different extracts against microbial strains, including Bacillus cereus, Klebsiella pneumoniae ATCC 13883, and Cryptococcus neoformans, were found to be 62.5 μg/ml, while Staphylococcus aureus (MRSA) has a MIC of 125 μg/ml with seed extract and 250 μg/ml with leaf extract. The other strains, including Escherichia coli, Salmonella typhimurium, and Pseudomonas aeruginosa, have a MIC of 500 μg/ml in both seed and leaf extracts. Schinus molle seeds and leaf exhibited considerable antioxidant action compared with ascorbic acid. Moreover, significant variation in the effects on Allium cepa root tips was observed upon treatment with Schinus molle seed and leaf extracts. The mitotic index in the group receiving Schinus molle seed and leaf extract showed a significant decrease when compared to the control cells. This decrease was based on the duration and dosage of exposure. On the other hand, there was a clear increase in the occurrence of chromosomal abnormalities. Finally, the findings showed that Schinus molle seed and leaf extract exhibit antibacterial, antioxidant, and anticytotoxic activities and revealed that they are a significant source of bioactive compounds with the possibility of use in biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Schinus molle (L.), commonly known as the Peruvian pepper tree or “pink pepper” due to the eatable red/pink seeds, is a plant species that belongs to the Anacardiaceae family. It is native to South America although it is distributed worldwide as an ornamental tree and has been traditionally used for medicinal purposes [1]. In conventional cooking, S. molle seeds have been employed as an alternative to black pepper and in the preparation of alcoholic beverages [2]. Many studies attribute some pharmacological properties to the S. molle tree as antifungal, antibacterial, antiviral, topical antiseptic, anti-inflammatory, antioxidant, anti-tumor, antispasmodic, analgesic, stimulant, and antidepressant characteristics [3,4,5,6]. Additionally, it has been used to treat rheumatism, respiratory and urinary tract infections, menstruation abnormalities, toothaches, and rheumatoid arthritis [7,8,9,10]. Moreover, studies’ findings have shown that essential oils from Schinus molle extracts have antibacterial and antioxidant effects [11,12,13,14].
Antioxidants have a crucial role in inhibiting the production of reactive nitrogen and oxygen species as well as their activities. It is thought that antioxidants can both prevent and treat different kinds of cancer. Natural antioxidant chemicals are being widely used in cancer prevention and treatment [15, 16]. Antioxidants are a potential treatment strategy when administered alone or in combination with antibiotics. Antioxidants have multiple applications in the fight against infectious diseases. They support the development or maintenance of robust immune cells to combat infections. Antioxidants also protect cells from harm brought on by pathogens. Furthermore, because antioxidants neutralize free radicals, which contribute to the pathogenesis, the course of disease is limited. Apart from mitigating the degrading and degenerative consequences of reactive species, several antioxidants exhibit antibacterial properties as well [17].
Gomes and colleagues used extracts from a plant matrix derived from the trash of the Brazilian pepper tree (Schinus terebinthifolia Raddi) processing industry chain to estimate antibacterial activity against MDR pathogens of clinical origin and conventional strains [18]. However, the Turchetti research group assessed the potential antibacterial activity of bioactive chemicals isolated from Schinus molle leaves against Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli), Gram-positive (Bacillus cereus, Staphylococcus aureus, Enterococcus faecalis), and Candida albicans [19]. Studies utilizing ethanolic, petroleum ether, and hexanic extracts of the leaves and seeds of Schinus molle found phytotoxic and insect repellant effects [20, 21].
Mice as models of animal tests were used in acute and subacute toxicity tests using ethanolic and hexanic extracts of Schinus molle leaves and seeds, demonstrating the safety of such tests when carried out under the protocol by Baser et al. [22]. The Allium cepa root tips test is vital because it serves as a good in vivo model, allowing for the prediction of eukaryote cytotoxicity. Previous studies showed that the essential oils of Schinus terebinthifolius and Schinus molle are mitodepressive and aneugenic inducers in onion and lettuce root meristems [23]. Moreover, Calotropis procera extract has cytogenetic effects on Vicia faba root tips [24]. Also, Owolarafe et al. examined the genotoxic and cytotoxic properties of leaf extracts from Ziziphus mauritiana on the root tips of Allium cepa [25]. The discovery of novel plant-based chemicals with cytotoxic effects may benefit from research on genotoxicity, mutagenicity, and cytogenetic effects [26, 27]. This information can be used to evaluate the safety of herbal extracts for human consumption and medicinal use. Hence, evaluating the phytochemical components, antimicrobial, antioxidant, and cytogenetic effects of Schinus molle extract on Allium cepa root tips is the goal of this study.
2 Materials and methods
2.1 Collection of Schinus molle seeds and leaves
Schinus molle seeds and leaf were collected in August 2022 from the Abha region in the south-west of Saudi Arabia. The leaves and ripped seeds of female trees of Schinus molle L. were previously cleaned with tap water, cut into small slices, and dried in the open air. After drying, each Schinus molle L. part was ground to a fine powder, which was used for the extraction. The collection and identification of the plant material was done by Shehata M. E.
2.2 Extraction of Schinus molle seeds and leaves
Different solvents were used to extract Schinus molle seeds and leaves, including hexane, ethyl acetate, methanol, and water, by soaking 10 g of Schinus molle seed or leaf powder in 100 ml of each solvent (Sigma-Aldrich, St. Louis, USA) for 24 h. After the soaking period, the extract underwent filtration using Whatman No. 1 filter paper. The resulting filtrate was subjected to evaporation in a vacuum evaporator at a temperature of 50 °C (Heidolph VV200, Schwabach, Germany). To facilitate further research, the extract was then stored in dark vials on ice at a temperature of −10 °C [28].
2.3 Phytochemical analysis of Schinus molle seed and leaf extracts by GC-MS
In accordance with Nagaraja et al. [29], gas chromatography-mass spectrometry (GC-MS) was employed to examine, quantify, and identify the main active constituents present in the extracts of Schinus molle seeds and leaves. To disperse the S. molle seed and leaf extracts, spectroscopy-grade methanol was utilized. The gas chromatography-mass spectrometry analysis was conducted using a Thermo Scientific trace GC1310-ISQ mass spectrometer located in Austin, TX, USA. A direct capillary column with a length of 30 m, a thickness of 0.25 μm, and an internal diameter of 25 mm was utilized. Helium was employed as the carrier gas at a split ratio of 1:30 to inject a 1 μl sample at 250 °C. The oven was initially preheated to 50 °C for 5 min, then ramped up to 230 °C at a rate of 5 °C per minute and held for 2 min. The mass spectrometer was set to scan in electron ionization mode, ranging from 40 to 1000 m/z, at a temperature of 200 °C and an electron energy of 70 eV. The obtained spectra of identified compounds were compared to those of known compounds listed in the WILEY 09 (Wiley, New York, NY, USA) and NIST 11 libraries.
2.4 Antimicrobial activity of Schinus molle seed and leaf extracts
Using Mueller-Hinton agar (MHA) for bacteria and potato dextrose agar (PDA) for yeast (HiMedia Laboratories Pvt. Ltd., India), the antimicrobial activity of different solvent extracts (hexane, ethyl acetate, methanol, and water) was examined. Clinical bacterial isolates (including Staphylococcus aureus (MRSA), Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhimurium) and the clinical unicellular fungus Cryptococcus neoformans, obtained from the bacteriology lab at Al-Azhar University’s Faculty of Science, were cultured overnight with a 0.5 McFarland turbidity standard. Additionally, standard strains of Staphylococcus aureus (ATCC 6538) and Klebsiella pneumoniae (ATCC 13883) were included. The bacterial cultures were spread on the surface of Mueller-Hinton agar (MHA), while the yeast cultures were spread on potato dextrose agar (PDA). Using a sterile cork borer, wells with a diameter of 6 mm were created on the agar plates. Then, 100 μl of each solvent extract was added to the respective wells. The plates were refrigerated at 4 °C for 2 h. Subsequently, the plates were incubated for 24 h at 37 °C for bacteria and 48 h at 28 °C for fungi. The controls used were amoxicillin for bacteria and fluconazole for yeast (HiMedia Laboratories Pvt. Ltd., India). The experiment was conducted in triplicate, and the inhibition zones were measured and recorded after incubation as mean ± standard error values [30].
2.5 Estimation of the MICs of Schinus molle seed and leaf extracts
By means of a broth microdilution assay, the MIC values of the promising extracts (methanolic extract) were determined against clinical isolates and a standard strain, according to Chaari et al. [31]. Different concentrations of extracts were prepared by performing double-fold dilution in the wells of a sterile microtiter plate. Each well contained 100 μl of double-strength Mueller-Hinton (MH) broth. The final concentrations of the extracts were 1000, 500, 250, 125, 62.5, 31.25, and 15.75 μg/ml. Except for the negative control well, all other wells received 20 μl of a cell suspension of microbial strains with a 0.5 McFarland standard. Positive control wells were filled with MH broth and a microbial suspension to test the broth’s ability to support bacterial growth. Sterility control was represented by wells containing sterile Mueller-Hinton broth. The plates were then incubated at 37 °C for 24 h. After the incubation period, bacterial growth was assessed by adding 30 μl of a resazurin solution (0.02% wt. v.) (HiMedia Laboratories Pvt. Ltd., India) to each well. The plate was re-incubated for an additional 3–8 h. A color change from blue to purple, red, or pink indicates proper growth of the microbes. In contrast, no color change was observed in the treated wells or the sterile control well, indicating the absence of microbial growth. The experiment was repeated, and the mean values were calculated.
2.6 Antioxidant activity
2.6.1 DPPH assay
To evaluate the antioxidant activity of the plant methanol extracts, the DPPH (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich, USA) method was employed. This method, previously described by Munteanu and Apetrei [32], was slightly modified for the current study. In short, the DPPH reagent was dissolved in MeOH (100 ml) at a concentration of 80 μl/ml. Specifically, 8 mg of the DPPH reagent was dissolved in 100 ml of MeOH. In a 96-well microplate (Thermo Fisher Scientific Inc, USA), 100 μl of the DPPH reagent was mixed with 100 μl of various concentrations of each compound (1000, 500, 250, 125, 62.5, 31.25, 15.62, and 7.81 g/ml). The mixture was then incubated at room temperature for 30 min. After the incubation period, the absorbance at 490 nm was measured using an ELISA reader (TECAN, Grodig, Austria). A control using 100 percent methanol was also included. The DPPH scavenging effect of each compound was quantified using the provided formula.
The antioxidant activity of the standard and each compound was measured as DPPH radical scavenging activity (%) and the IC50 DPPH values (sample concentration required to inhibit 50% of DPPH radicals) were calculated.
2.6.2 ABTS assay
ABTS (2,2 (3-ethylbenzothiazoline-6-sulfonic acid) was purchased from Sigma-Aldrich (Sigma-Aldrich Co. Louis St., MO, USA)) is another assay for evaluating antioxidant activity. It was used to assess the ability of methanolic extract to scavenge the free radical produced from the ABTS reagent, as described by Ilyasov et al. [33]. The same concentrations examined were tested in this assay. The method used to evaluate the antioxidant activity of different compounds using the ABTS assay. The antioxidant activity of the standard and methanol extracts was calculated as ABTS scavenging activity (%) using the following equation:
The IC50 ABTS values (sample concentration required to inhibit 50% of ABTS radicals) were calculated.
2.7 Cytological investigations
The seedlings of Allium cepa were acquired from the onion research department of the Field Crops Research Institute, which is located at the Agricultural Research Center in Giza, Egypt. A. cepa seeds were germinated in distilled water until the root length reached approximately 1 cm. The roots were treated with seed and leaf extracts derived from Schinus molle for durations of 6, 12, and 24 h, with three replicates for each experimental condition. Twenty-four hours were spent preserving the roots in a solution containing ethanol and acetic acid in a ratio of 3:1. Subsequently, hydrolysis of the roots was carried out by immersing them in water at a temperature of 60 °C for a period of 10 min, using a solution of 1 N HCl. Subsequently, the root tips were subjected to the Feulgen squash procedure, as described by Özkara et al. [34]. Using an optical microscope (Poland) with a 40× magnification, the mitotic index and chromosomal abnormalities were evaluated on a sample of at least 2000 cells from both the control group and all treatment groups. The mitotic index is calculated as the percentage of dividing cells relative to the total number of observed cells. The phase index% is calculated by dividing the number of cells in each phase by the total number of observed dividing cells. The proportion of chromosomal abnormalities (CA), the ratio of cells with chromosomal modifications to the total number of cells in division multiplied by 100.
2.8 Statistical analysis
Minitab 18 supplemented with a statistical tool and Microsoft Excel were used to determine the inhibition zone as the mean SD value. Mitotic index and chromosomal anomaly frequency differences between treatments and controls were analyzed statistically. One-way ANOVA (Sigma Plot 13.0 software) and SPSS were used for statistical analysis, and significance thresholds of p ≤ 0.05 and p ≤ 0.01 were calculated.
3 Results and discussion
3.1 Phytochemical analysis of Schinus molle extracts by GC-MS spectroscopy
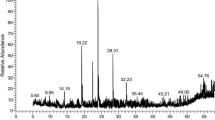
GC-MS analysis provides a comprehensive spectral profile of the compounds present in the samples under investigation. As a result, GC-MS has gained significant recognition in recent years as a key technological tool for identifying and characterizing secondary metabolites in various organisms, including both plants and non-plant species [19]. Table 1 and Fig. 1A and B show a phytochemical analysis of compounds found in seed and leaf extracts. The results revealed that Schinus molle seed extract contains seven peaks with a major compound, bis (2-ethylhexyl) phthalate, at 59.11%, followed by n-hexadecanoic acid at 10.84, while Schinus molle leaf extract exhibits sixteen peaks with a main compound, squalene, at 16.87%, followed by azulene at 14.88% and lupeol at 12.4%. In a previous study by Turchetti et al. [19], it reported that elemol is a major compound with a percentage (46.28%), followed by germacrene D (18.10%), which was the main sesquiterpene family component that was identified; nevertheless, sabinene was found to be the main terpene group component (1.65%). Squalene was present and reached a noteworthy 10.60%. Thus, the difference in chemical constituents due to the composition of S. molle may vary by seasons and places from which the plant material was obtained, as well as by solvents and portions (leaves or seeds) of the plant employed for the extraction [19]. Moreover, Deveci et al. [49] found germacrene D as a significant ingredient in S. molle leaf hexane extract. The biological activity of Schinus molle seed and leaf extracts was recorded in Table 1, which also includes information on the activity of the compounds and their references. The compounds were searched for their antimicrobial, anti-inflammatory, antibacterial, anti-ulcer, anti-cancer, antiallergic, anti-tumor, anti-oxidative, antiviral, antifungal, larvicidal, hemolytic, pesticide, favor, antioxidant, anticancer, anti-diabetic, cardio, and hepatoprotective activities [35,36,37,38,39].
3.2 Antimicrobial activity of Schinus molle seed and leaf extracts
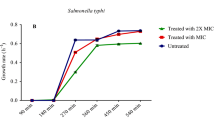
Schinus molle seed and leaf extracts were assayed against clinical strains of Staphylococcus aureus (MRSA), Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, and Cryptococcus neoformans, in addition to the standard strains of Staphylococcus aureus ATCC 6538 and Klebsiella pneumoniae ATCC 13883. The results in Tables 2 and 3 and Fig. 2a and b show that Schinus molle seeds extracted with methanol exhibited strong activity against tested microorganisms including Gram-positive and Gram-negative bacteria and fungi compared with other solvents and S. molle leaf extract with inhibition zones ranging from 15 ± 0.57 to 27.33 ± 0.88 mm. Several studies reported the antimicrobial activity of Schinus molle seeds and leaves [10,11,12,13,14, 49]. Also, Turchetti and colleagues reported the antibacterial activity of bioactive chemicals separated from Schinus molle against Gram-positive (Staphylococcus aureus, Enterococcus faecalis, Bacillus cereus), Gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli), and Candida albicans [19]. With mean inhibition zones of 11, 10, and 10 mm, respectively, the leaf essential oils of Schinus molle were more efficient against Staphylococcus epidermidis, Staphylococcus aureus, and Enterococcus faecalis. Leaf and fruit essential oils of Schinus molle exhibit antibacterial activity against antibiotic-resistant Salmonella enteritidis serovar Typhimurium and Pseudomonas aeruginosa [50].
a Antimicrobial activity of various solvent extracts Schinus molle seeds (1 = water, 2 = methanol, 3 = ethyl acetate, 4 = hexane) against clinical and standard strain. D = negative control (DMSO), C = positive control (amoxicillin for bacteria and fluconazole for unicellular fungi Cryptococcus neoformans). b Antimicrobial activity of various solvent extracts of Schinus molle leaves (1 = water, 2 = methanol, 3 = ethyl acetate, 4 = hexane) against clinical and standard strain. D = negative control (DMSO), C = positive control (amoxicillin for bacteria and fluconazole for unicellular fungi Cryptococcus neoformans)
3.2.1 Minimum inhibitory concentrations (MICs) of Schinus molle seed and leaf extracts
The research on natural compounds and plant extracts are actively researched for their antimicrobial properties and have potential applications in medicine, agriculture, and food preservation. While there are still many challenges to overcome, the use of natural compounds offers a promising alternative to conventional antibiotics [29]. Table 4 shows the minimum inhibitory concentrations (MICs) of different extracts (seed and leaf extracts) against microbial strains. The MICs is the lowest concentration of an antimicrobial agent that inhibits the visible growth of a microorganism after overnight incubation. The results indicate that both extracts have the lowest MIC values of 62.5 μg/ml against Bacillus cereus, Klebsiella pneumoniae ATCC 13883, and Cryptococcus neoformans. Staphylococcus aureus (MRSA) has a MIC of 125 μg/ml when using seed extract and 250 μg/ml with leaf extract. The other strains, Escherichia coli, Salmonella typhimurium, and Pseudomonas aeruginosa, have a MIC of 500 μg/ml against both seed and leaf extracts. Staphylococcus aureus ATCC 6538 has a MIC of 250 μg/ml with seed extract and 500 μg/ml with leaf extract. The results revealed that the seed and leaf extracts have antimicrobial properties, but their effectiveness varies depending on the microbial strain. In a previous study, fruit and leaf essential oils of Schinus molle exhibit high antibacterial activity against Staphylococcus epidermidis and Staphylococcus aureus, with MICs of 63 and 125 μg/ml for leaf essential oils and 125 and 500 μg/ml for fruit essential oils, respectively [50]. They did, however, exhibit only moderate efficacy against other Gram-negative bacteria, including Salmonella enteritidis serovar Typhimurium, Proteus mirabilis, and Pseudomonas aeruginosa, with MICs >1000 μg/ml. Additionally, S. molle essential oils showed efficacy against fungi that cause food to deteriorate. While leaf essential oils showed better activity against Rhizopus stolonifer (500 μg/ml), Aspergillus japonicus, and Rhizopus oryzae (750 μg/ml), fruit essential oil was more effective against Aspergillus japonicus (250 μg/ml) and Aspergillus niger (750 μg/ml) [50]. Also, Turchetti and colleagues [19] demonstrated the antimicrobial activity of S. molle extract with inhibition zones between 8 and 17 mm against Staphylococcus aureus, Enterococcus faecalis, Candida albicans, and Bacillus subtilis. A minimal inhibitory concentration (MIC) assay of the tested extracts ranged from 25 to 400 μg/ml. In addition, the most effective residues against S. aureus (MIC 0.60–0.90 mg/ml), Enterococcus faecium, and Enterococcus faecalis (MIC 1.20–2.10 mg/ml) were the methanolic fraction and the hydroethanolic extract [19].
3.3 Antioxidant activity
To assess their antioxidant properties, in vitro studies were performed on Schinus molle seed and leaf extracts using DPPH and ABTS radical-scavenging assays. Free radical DPPH can accept an electron or a hydrogen ion to transform into a stable diamagnetic molecule. The drop in absorbance brought on by plant antioxidants was used to gauge the DPPH radical’s capacity for reduction. The antioxidant activity of a methanolic extract of Schinus molle seeds and leaves was investigated at various concentrations (1000–7.81 μg/ml). The result obtained from DPPH radical scavenging activity revealed that ascorbic acid exhibits the highest antioxidant activity among the three antioxidants tested, with a maximum scavenging activity of 95.79% at a concentration of 1000 μg/ml. Seed and leaf extracts exhibit scavenging activity, with maximum values of 78.32% and 75.46%, respectively, at a concentration of 1000 μg/ml as shown in Fig. 3A. The results of the antioxidant activity of ascorbic acid, seed, and leaf extracts at different concentrations using ABTS show that highest concentration of 1000 μg/ml of ascorbic acid has the highest antioxidant activity of 93.55%, followed by seed extract with 87.94% and leaf extract with 84.146%. At the lowest concentration of 7.81 μg/ml, ascorbic acid still has the highest antioxidant activity of 52.787%, while seed extract has the lowest activity of 1.789% and leaf extract has 4.39% as shown in Fig. 3B. Thus, the result is consistent with previous studies that have shown that ascorbic acid has strong antioxidant activity [29, 33].
The results obtained revealed that seed and leaf extracts have strong antioxidant activity compared to ascorbic acid. Overall, the DPPH assay is a useful method for evaluating the antioxidant activity of different compounds. However, it is important to note that the assay measures only one aspect of antioxidant activity and may not reflect the overall antioxidant capacity of a compound. Therefore, it is important to use multiple assays to evaluate the antioxidant activity of different compounds, and so we use another method with the DPPH method. Aqueous root extract from Schinus molle L. produced the highest antioxidant activity when tested using the FRAP and DPPH assays (54.41 μg·ml−1 and 207.42 μg·ml−1 of extracts, respectively) [51]. As measured by IC50 values with the DPPH technique, the methanol extracts of Schinus molle seeds, leaves, and ascorbic acid exhibit IC50 values of 25.5, 100.5, and 8.0 μg/ml, while the ABTS assay exhibits IC50 values of 22.8, 98.0, and 7.2 μg/ml, respectively. Natural products’ ability to create stable radicals is typically linked to their capacity for radical scavenging. Reactive oxygen species are substances created as a consequence of biological activity that are prevented by antioxidants [50]. Additionally, antioxidants can balance out free radicals, which are responsible for a number of diseases. Antioxidants are thought to have antibacterial, anticarcinogenic, anticancer, anti-inflammatory, and anti-atherosclerotic properties [33].
3.4 Cytotoxicity effects of Schinus molle seed and leaf extracts
3.4.1 Cytotoxicity of the plant extracts on percentage mitotic index
Figure 4A illustrates the change in the mitotic index (MI) percentage in Allium cepa root tips following treatment with extracts from Schinus molle leaves and seeds at various time intervals (6 h, 12 h, and 24 h), relative to the control group. The findings demonstrate that both leaf and seed extracts of S. molle resulted in a reduction in the mitotic index (MI) percentage in the root tips of A. cepa when compared to the control group. The reduction in MI was in correlation with increasing concentrations of the extract and more significant due to seed extract than leaf extract and the percentage of MI reduces as the exposure period to the extracts increases. According to the research, the extracts have a distinguished impact on the cell cycle of A. cepa root tips. The outcomes of this study suggest that the extracts obtained from the leaves and seeds of pink pepper exhibit a significant effect on the cell cycle of Allium cepa root tips. These findings are consistent with earlier research on the cytotoxic and genotoxic effects of plant extracts on the root tips of A. cepa [1, 52, 53]. The reduced MI in A. cepa roots treated with S. molle leaf as well as seed extracts could be caused by either disruption in the cell cycle or chromatin dysfunction brought on by an external factor, in this case, DNA interactions with S. molle leaf and seed extracts. Inamdar et al. [53] conducted a study to assess the cytotoxicity potential of different extracts derived from the leaves of Ziziphus mauritiana (Lam). According to the scientists’ observations using the Allium cepa model, the percentage of mitotic index (%MI) was reduced, and there was a dose-related reduction of the root tip length [52, 54]. In a study conducted by Santos et al. [54], the authors examined the cytotoxic and genotoxic effects of leaf extracts from Inula viscosa on the root meristem cells of Allium cepa.
The evaluation of the effect of extracts on the phase index revealed that the control group had a prophase percentage of 53.75%. However, at all given time points, the root tips of A. cepa subjected to an extract derived from S. molle seeds displayed a lower proportion of cells in the prophase stage than the untreated group. At the 12-h mark, the lowest percentage observed was 12.52%. In comparison to the untreated group, applying pink pepper leaf extract to the root tips of A. cepa resulted in a decrease in the proportion of cells in the prophase stage over the course of all observed time intervals. The minimum percentage, which was 17.33%, was seen at the 12-h point (Fig. 4B and C). The cellular effects of the S. molle seed extract exhibit a greater degree of significance in relation to the S. molle leaf extract, specifically in the prophase and telophase phases of the cell cycle (Fig. 4B and C). Furthermore, it was observed that the control group showed a metaphase percentage of 24.50%. On the other hand, when A. cepa root tips were subjected to pink pepper seed extract, a significantly higher metaphase percentage was found in comparison to the control group during all recorded time periods. Importantly, a 12-h period of treatment produced the highest recorded metaphase percentage (42.11%). The results of the study reveal that the application of a S. molle leaf extract to the root tips of A. cepa led to a greater percentage of metaphase cells in comparison to the control group, consistently seen throughout all time intervals. The results of the study revealed that the extracts led to a remarkable decrease in the mitotic index and produced chromosomal abnormalities [1, 55]. The current study’s outcomes indicate that the impact of the S. molle seed extract on the cell cycle is more significant in comparison to the S. molle leaf extract, particularly during the prophase and telophase stages.
The highest observed percentage, which reached 41.95%, was recorded at the 12-h mark (Fig. 4B and C). The untreated group showed an anaphase proportion of 8.45%. Conversely, when A. cepa root tips were treated with S. molle seed extract, a significantly higher percentage of anaphase was found compared to the control group at all recorded time intervals. The largest observed proportion of anaphase, which was 30.33%, was recorded after 24 h of treatment. In comparison to the control group, the application of S. molle leaf extract to the root tips of A. cepa significantly increased the proportion of cells in the anaphase stage during the course of all recorded time intervals. The greatest observed percentage, which reached 30.71%, was verified following a 12-h duration of treatment (Fig. 4B and C). In the untreated group, the telophase proportion remained stable at 13.28%. However, it was seen that A. cepa root tips treated with S. molle seed extract had a greater telophase percentage than the untreated group at all time intervals. The telophase percentage reached its peak at 33.07% within a 24-h period. The telophase % of A. cepa root tips that were exposed to treatment with pink pepper leaf extract consistently displayed lower values compared to the control group during all recorded time intervals. At the 24-h mark, the telophase percentage reached its lowest value of 8.02% (Fig. 4B and C). The findings indicate that, in comparison to the control group, the extracts made from the leaves and seeds of S. molle have a substantial impact on the cell cycle of A. cepa root tips. The extracts possess the ability to produce chromosomal aberrations and interrupt the mitotic process in the root tips. The cellular effects of the S. molle seed extract show a greater degree of significance in relation to the pink pepper’s leaf extract, specifically in the prophase and telophase phases of the cell cycle. On the other hand, it is obvious that the leaf extract of S. molle has a greater impact on the metaphase and anaphase stages than the seed extract does (Fig. 4C). The observation in this study lines up with the findings of Santos et al. [54], who tested the cytotoxic and genotoxic effects of Inula viscosa leaf extracts on Allium cepa root meristem cells. In contrast, the effect of S. molle leaf extract on the metaphase and anaphase stages has a greater impact than that of S. molle seed extract. This result in agreement with the outcomes of a study conducted by Ramos et al., which examined the ultrastructure of Anacardium occidentale L. leaves from the Amazon region in northern Brazil using scanning microscopy [55]. Concurrently, Pawlowski et al. [23] reported that their research findings demonstrated a decrease in the mitotic index of onion and lettuce by 21.05% and 82.03%, respectively, when exposed to essential oils produced from S. molle and S. terebinthifolius. The findings of both experiments indicate that the extracts obtained from Schinus molle have a significant impact on the mitotic index of plant cells [23].
3.4.2 Percentage abnormality
As illustrated in Fig. 5A, the percentage of abnormality index (Ab%) in the root tips of Allium cepa plants treated with extracts derived from Schinus molle leaves and seeds is compared to the untreated group. The extracts from S. molle seeds and leaves resulted in a higher Ab% compared to the control group across all observed time intervals. Notably, a maximum Ab% of 90.9% was observed after 24 h of treatment with seed extract (Fig. 5A). Figure 5B and C present the phase abnormality index, which offers insights into the proportion of abnormality (Ab%) observed during various cell-cycle phases in the root of Allium cepa. In the region of Ab prophase %, it is obvious that the control group had the lowest Ab prophase % throughout all the recorded time intervals. The Ab prophase % of the root tips of A. cepa that were treated to an extract from S. molle seeds was found to be considerably higher compared to the control group during all observed time periods. Significantly, the largest proportion of 22.0% was found after a 6-h treatment period. The Ab prophase % of the root tips of A. cepa, which were treated with an extract obtained from the leaves of S. molle, demonstrated a statistically significant increase in comparison to the control group throughout all recorded time periods. Following a 6-h treatment, the largest proportion was recorded, totaling 20.67% (Fig. 5B and C). Regarding irregular cells % all mitotic phases (prophase, metaphase, telophase, and anaphase), extract made from pink pepper seeds and leaves exhibited a significant higher percentage of abnormal root tip cells compared to the control group across all observed time periods (Fig. 5B and C). The results imply that the extracts derived from the leaves and seeds of Schinus molle significantly impact the development of chromosomal abnormalities and disturbances in mitotic processes in the root tips of A. cepa. The highest proportion, amounting to 39.8%, was observed following a treatment duration of 12 h (Fig. 5B and C). The results of this study are consistent with previous reports that have studied the cytogenetic and genotoxic impacts of botanical extracts on the root tips of A. cepa. Nefic et al. [56] conducted research on the chromosomal and nuclear modifications caused by alprazolam in root tip cells of A. cepa. The study revealed alterations in both the structure and quantity of chromosomes during the process of mitosis.
The observed effects of the S. molle seed extract on chromosomal abnormalities and mitotic disruptions are more obvious in comparison to those of the S. molle leaf extract. The root tips of A. cepa, when exposed to S. molle seed extract, had a considerably higher percentage of Ab anaphase compared to the control group throughout all observed time intervals. Significantly, a peak percentage of 30.3% was seen following a 24-h duration of treatment. Schinus molle leaf extract was applied to the root tips of A. cepa, resulting in a significantly larger percentage of Ab anaphase than the control group at all observed time intervals. It is significant to point out that after 12 h of treatment, the highest percentage recorded was 25.4% (Fig. 5B and C). Regarding telophase, it was found that, throughout all recorded time intervals, the control group had the lowest percentage of aberrant telophase occurrences. The experimental group, consisting of A. cepa root tips treated with a pink pepper seed extract, showed a substantially higher percentage of Ab telophase compared to the control group during all observed time periods. The highest documented proportion, which reached 33.0%, was observed following a 24-h period of treatment. The root tips of Allium cepa were subjected to leaf extract derived from S. molle. As a result, there was a notable decrease in the percentage of Ab telophase in comparison to the control group in all the observed time intervals. The minimum recorded percentage observed was 7.39% after a 24-h period of treatment (Fig. 5B and C). The results suggest that the extracts obtained from the leaves and seeds of pink pepper have a significant effect on the emergence of chromosomal aberrations and disruptions in mitotic processes in the root tips of A. cepa. The observed effects of the S. molle seed extract on chromosomal abnormalities and mitotic disruptions are more significant in comparison to those of the S. molle leaf extract. This is supported by the consistently higher percentage of abnormalities (Ab%) reported throughout all recorded time periods. On the other hand, it was noticed that the effect of the S. molle leaf extract on the Ab Pro % and Ab Meta % was more significant in comparison to the S. molle seed extract (Fig. 5D). In a study carried out by Inamdar et al. [53], the cytotoxic properties of different extracts obtained from Ziziphus mauritiana (Lam) leaves were investigated using the Allium cepa model. The results indicated a decrease in the percentage of mitotic index (%MI) and a reduction in the length of root tips, which correlated with the concentration of the extracts. Additionally, Garzoli et al. found that S. molle leaf extracts prepared using successive extraction with solvents of different polarity exhibited potential cytotoxic activity against the HL-60 human leukemia cell line [57].
3.4.3 Chromosomal abnormalities
The obtained data show that the extracts of the leaves as well as seeds of Schinus molle exhibit a considerable impact on the induction of chromosomal abnormalities and mitotic disturbances in the root tips of Allium cepa. The most common chromosomal abnormalities identified were stickiness, irregularity, C-metaphase, forward, lagging, and disrupted chromosomes. The examination of chromosomal anomalies in A. cepa indicated that stickiness was the most prevalent aberration, exhibiting a significant frequency throughout various phases of cell division, as seen in Figs. 5D and 6. It was found that the S. molle seed extract produced a higher proportion of chromosomal aberrations than the S. molle leaf extract at all time intervals. The given Fig. 6 shows that S. molle seed as well as leaf extracts caused various forms of mitotic abnormal cells in the roots of the A. cepa. Numerous different factors could contribute to chromosomal abnormalities. Kumari et al. [58] reported that numerous other genetic endpoints occur in treated plant systems with chemicals, such as changes in ploidy, chromosomal abnormalities, and sister chromatid exchanges. The occurrence of chromosomal abnormalities can be attributed to a variety of factors. Chemical interference has an enormous effect during DNA repair. The existence of sticky chromosomes, a sign of harmful effects on chromosome structure, was seen as a common abnormality in the root tips of plants exposed to these extracts during the metaphase, anaphase, and telophase stages. This finding offers a different viewpoint from the research done by [59,60,61]. These investigations demonstrated that DNA fragmentation levels were substantially higher in the comet assays performed using A. cepa tests, so that chromosomal stickiness is recognized as the predominant chromosomal aberration [62,63,64]. Moreover, the enzymatic system experienced disturbance due to the adhesive properties, resulting in a slowing down of cellular division [61]. The phenomenon of sticky chromosomes is thought to be confirmed by the remarkable rise in DNA fragmentation [55, 59].
Different types of chromosomal abnormalities caused by Schinus molle seed and leaf extracts in Allium cepa: A–B vacuolated nucleus at prophase; C–D disturbed and C-metaphase; E–F sticky metaphase; G–H sticky and forward chromosome at anaphase; I–J anaphase with bridges; K–L forward chromosomes at anaphase; M–N diagonal anaphase; and O–P sticky telophase
According to Nwakanma and Okoli [62], stickiness is a characteristic that signifies elevated toxicity and can lead to aberrant protein-protein interactions. The adhesive properties and various chromosomal aberrations observed in our study may be clarified by the interaction between the genotoxic secondary metabolites of S. molle extracts with DNA and proteins, which causes harmful changes to their physical and chemical properties. Other possible explanations include the condensation of chromatin in the nucleus or the formation of crosslinks within and between chromatids. Inhibition of DNA synthesis or an impairment in the G2 phase of the cell cycle, which prevents the cell from initiating mitosis, could be the cause of a decline in mitotic activity [61]. According to the findings of the research, it is advisable to assess micronuclei exclusively in cells that have undergone mitosis either during exposure to the test substance or in the subsequent period after exposure [63,64,65]. The lack of micronuclei observed in this study could possibly be explained by the short exposure time, which might have prevented the development of micronuclei. If the exposure time were prolonged as suggested by [66, 67], micronuclei can be observed in the root tips of A. cepa. The exact ways in which extracts from S. molle may affect the cell cycle in Allium cepa root tips are not fully understood. Nevertheless, earlier research has indicated that compounds like alkaloids, flavonoids, and terpenoids, which are secondary metabolites, could potentially be accountable for the harmful effects of plant extracts on cellular structures, causing cytotoxicity and genotoxicity. These substances may disrupt various biological processes, including DNA replication, transcription, and cell division, ultimately resulting in chromosomal irregularities and cell death. Earlier studies have suggested that the observed cytotoxic and genotoxic impacts of plant extracts on cellular structures might be attributed to the existence of secondary metabolites, including alkaloids, flavonoids, and terpenoids [1, 55]. These substances have the capacity to interfere with a variety of biological processes, such as DNA replication, transcription, and cell division, leading to chromosomal abnormalities and cellular death. Finally, chromosomal aberrations can be linked to a variety of factors, including genetic effects, environmental factors, and anthropogenic influences. The presence of these abnormalities has the potential to impact the overall health and growth of the plant through the disruption of multiple cellular processes, impairment of meiosis, and alteration of reproductive success in hybrid plants [64, 65].
The outcomes of this study show that the extracts from Schinus molle leaves and seeds have a considerable effect on the cell cycle of Allium cepa root tips, which advances our understanding of how plant extracts affect the cell cycle. These effects trigger chromosomal abnormalities and disrupt the process of mitosis in the root tips. The study also emphasizes how Schinus molle seed and leaf extracts affect the cell cycle differently at various stages, which could contribute to future research on the processes behind the cytotoxic and genotoxic effects of plant extracts on cells.
4 Conclusion
This study investigated the phytochemical constituents, antimicrobial, antioxidant, and cytotoxic activities of Schinus molle (L.) seed and leaf extracts. The seed extract contained bis (2-ethylhexyl) phthalate as the major compound, while the leaf extract contained squalene as the main compound, along with azulene and lupeol. The extracts of Schinus molle leaves and seeds demonstrated notable antibacterial and antioxidant effects against Gram- and Gram-positive bacteria as well as fungi that cause food spoilage. Due to these effects, they may be useful in the biotechnology, food, and/or pharmaceutical industries. In terms of cytotoxicity, the extracts caused a decrease in the mitotic index and an increase in the occurrence of chromosomal abnormalities in Allium cepa root tips. These findings highlight the antibacterial, antioxidant, and cytotoxic properties of Schinus molle seeds and leaves, indicating their potential use in biomedical research and agriculture as a source of bioactive compounds. Further studies such as the use of other separation and purification methods of the bioactive compounds of Schinus molle extracts and in vivo investigations might be helpful in clarifying the mechanism of action of the noted positive benefits.
Data availability
Not applicable.
References
Aşkin Çelik T, Aslantürk ÖS (2010) Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. J Biomed Biotechnol [Internet] 2010:1–8. https://doi.org/10.1155/2010/189252
Marongiu B, Alessandra PSP, Casu R, Pierucci P (2004) Chemical composition of the oil and supercritical CO2 extract of Schinus molle L. Flavour Fragance J 19:554–558
Duke J (1985) Handbook of medicinal herbs. CRC Press, Boca Raton. Florida. EDQM, 2007. European pharmacopoeia, 7th edn. Directorate for the Quality of Medicines and HealthCare of the Council of Europe, Strasbourg (FR)
Alanís-Garza BA, González-González GM, Salazar-Aranda R, Torres NWD, Rivas-Galindo VM (2007) Screening of antifungal activity of plants from the northeast of Mexico. J Ethnopharmacol 114:468–471
Machado DG, Kaster MP, Binfaré RW, Dias M, Santos ARS, Pizzolatti MG, Brighente IMC, Rodrigues ALS (2007) Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: evidence for the involvement of the monoaminergic system. Prog Neuro-Psychopharmacol Biol Psychiatry 31:421–428
Guala M, Elder H, Perez G, Chiesa A (2009) Evaluación del poder antioxidante de fracciones de aceite esencial crudo de Schinus molle L. obtenidas por destilación al vacío. Inf Tecnol 20:83–88
Perez C, Anesini C (1994) Inhibition of Pseudomonas aeruginosa by Argentinean medicinal plants. Fitoterapia 65:169–172
Barrachina MD, Bello R, Martinezcuesta MA, Primoyufera E, Espulgues J (1997) Analgesic and central depressor effects of the dichloromethanol extract from Schinus molle L. Phytother Res 11:317–319
Marzouk M, Moharram F, Haggag E, Ibrahim M, Badary O (2006) Antioxidant flavonol glycosides from Schinus molle. Phytother Res 20:200–205
Atti dos Santos AC, Rossato M, Serafifini LA, Bueno M, Crippa LB, Sartori VC, Dellacassa E, Moyna P (2010) Antifungal effect of Schinus molle L., Anacardiaceae, and Schinus terebinthifolius Raddi, Anacardiaceae, essential oils of Rio Grande do Sul. Braz J Pharmacogn 20:154–159
Dikshit A, Naqvi AA, Husain A (1986) Schinus molle: a new source of natural fungitoxicant. Appl Environ Microbiol 51:1085–1088
Hayouni EA, Chraief I, Abedrabba M, Bouix M, Leveau JY, Mohammed H, Hamdi M (2008) Tunisian Salvia officials L. and Schinus molle L. essential oils: their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int J Food Microbiol 125:242–251
Murray A, Gurovic M, Rodriguez S, Murray M, Ferrero A (2009) Acetylcholinesterase inhibition and antioxidant activity of essential oils from Schinus areira L. and Schinus longifolia (Lindl.) Speg. Nat Prod Commun 4:873–876
Salazar-Aranda R, Pérez-López LA, López-Arroyo J, Alanís-Garza BA, Torres NWD (2011) Antimicrobial and antioxidant activities of plants from northeast of Mexico. Evid Based Complement Alternat Med 2011:6
Dastmalchi N, Baradaran B, Latifi-Navid S, Safar Alizadeh R, Ma KS, Amini M, Elmira RE, Lotfinejad P (2020) Antioxidants with two faces toward cancer. Life Sci 258:118186
El-Sherbiny GM, Gazelly AM, Sharaf MH, Moghannemm SA, Shehata M, Ismail MKA, El-Hawary AS (2022) Exploitation of the antibacterial, antibiofilm and antioxidant activities of Salvadora persica (Miswak) extract. J Bioresour Bioprod. https://doi.org/10.1016/j.jobab.2022.11.006
Kaur J, Kaur R, Kaur A (2018) Dietary antioxidants and infectious diseases. In: Singh P (ed) Infectious diseases and your health. Springer, Singapore. https://doi.org/10.1007/978-981-13-1577-0_16
De Gomes RBA, de Souza ES, Gerhardt Barraqui NS, Tosta CL, Nunes APF, Schuenck RP, Ruas FG, Ventura JA, Filgueiras PR, Kuster RM (2020) Residues from the Brazilian pepper tree (Schinus terebinthifolia Raddi) processing industry: chemical profile and antimicrobial activity of extracts against hospital bacteria. Ind Crop Prod 143:111430
Turchetti G, Garzoli S, Laghezza Masci V, Sabia C, Iseppi R, Giacomello P, Tiezzi A, Ovidi E (2020) Antimicrobial testing of Schinus molle (L.) leaf extracts and fractions followed by GC-MS investigation of biological active fractions. Molecules 25:1977. https://doi.org/10.3390/molecules25081977
Abdel-Sattar E, Zaitoun AA, Farag MA, Gayed SHE, Harraz FMH (2010) Chemical composition, insecticidal and insect repellent activity of Schinus molle L. leaf and fruit essential oils against Trogoderma granarium and Tribolium castaneum. Nat. Prod. Res.: Former. Nat Prod Lett 24:226–235
Zahed N, Hosni K, Brahim NB, Kallel M, Sebei H (2010) Allelopathic effect of Schinus molle essential oils on wheat germination. Acta Physiol Plant 32:1221–1227
Baser KHC, Kürkçüoglu M, Demirçakmak B, Uülker N, Beis SH (1997) Composition of the essential oil of Schinus molle L. grown in Turkey. J Essent Oil Res 9:693–696
Pawlowski Â, Kaltchuk-Santos E, Zini CA, Caramão EB, Soares GLG (2012) Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): mitodepressive and aneugenic inducers in onion and lettuce root meristems. S Afr J Bot [Internet] 80:96–103. https://doi.org/10.1016/j.sajb.2012.03.003
Haroun SA, Shehri AMA (2001) Cytogenetic effects of Calotropis procera extract on Vicia faba L. Cytologia (Tokyo) [Internet] 66(4):373–378. https://doi.org/10.1508/cytologia.66.373
Owolarafe TA, Salawu K, Ihegboro GO, Ononamadu CJ, Alhassan AJ, Wudil AM (2020) Investigation of cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam) leaf Allium cepa model. Toxicol Rep [Internet] 7:816–821. https://doi.org/10.1016/j.toxrep.2020.06.010
Yuan B, Li S, Xiong T, Ting. (2019) Cytogenetic and genotoxic effects of Ipomoea cairica (L.) sweet leaf aqueous extract on root growth of Allium cepa var. agrogarum (L.). Allelopathy J [Internet] 46(2):61–70. https://doi.org/10.26651/allelo.j/2019-46-2-1209
Abdelrahman M, Mahmoud HYAH, El-Sayed M, Tanaka S, Tran LS (2017) Isolation and characterization of Cepa2, a natural alliospiroside A, from shallot (Allium cepa L. Aggregatum group) with anticancer activity. Plant Physiol Biochem [Internet] 116:167–173. https://doi.org/10.1016/j.plaphy.2017.05.006
Phiri BN, Eniah Lemogang Serame EL, Pheko T (2021) Extraction, chemical composition, and antioxidant activity analysis of essential oil from Schinus molle medicinal plant. Am J Essential Oils Nat Prod 9(4):01–09
Nagaraja SK, Nayaka S, Kumar RS (2023) Phytochemical analysis, GC–MS profiling, and in vitro evaluation of biological applications of different solvent extracts of Leonotis nepetifolia (L.) R.Br. flower buds. Appl Biochem Biotechnol 195:1197–1215. https://doi.org/10.1007/s12010-022-04201-2
Martins MR, Arantes S, Candeias F, Tinoco MT, Cruz-Morais J (2014) Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 151(1):485–49. https://doi.org/10.1016/j.jep.2013.10.063
Chaari M, Elhadef K, Akermi S et al (2022) Multiobjective response and chemometric approaches to enhance the phytochemicals and biological activities of beetroot leaves: an unexploited organic waste. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-03645-0
Munteanu IG, Apetrei C (2021) Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci 22(7):3380. https://doi.org/10.3390/ijms22073380
Ilyasov IR, Beloborodov VL, Selivanova IA, Terekhov RP (2020) ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int J Mol Sci 21(3):1131. https://doi.org/10.3390/ijms21031131
Özkara A, Akyıl D, Erdoğmuş SF, Konuk M (2011) Evaluation of germination, root growth and cytological effects of wastewater of sugar factory (Afyonkarahisar) using Hordeum vulgare bioassays. Environ Monit Assess [Internet] 183(1–4):517–524. https://doi.org/10.1007/s10661-011-1936-7
Shoaib M, Aygun AI, Ganbarov K (2019) Cyclohexane and its functionally substituted derivatives: important class of organic compounds with potential antimicrobial activities. J Microbiol Biotechnol Food Sci 9(1):84
Peet J, Selyutina A, Bredihhin A (2016) Antiretroviral (HIV-1) activity of azulene derivatives. Bioorg Med Chem 24(8):1653–1657
Bakun P, Czarczynska-Goslinska B, Goslinski T, Lijewski S (2021) In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med Chem Res 30:834–846
Khodarahmi G, Asadi P, Hassanzadeh F, Khodarahmi E (2015) Benzofuran as a promising scaffold for the synthesis of antimicrobial and antibreast cancer agents: a review. J Res Med Sci official J Isfahan Univ Med Sci 20(11):1094
Miao YH, Hu YH, Yang J, Liu T, Sun J, Wang XJ (2019) Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv 9(47):27510–27540
Perigo CV, Torres RB, Bernacci LC, Guimaraes EF, Haber LL, Facanali R, Marques MOM (2016) The chemical composition and antibacterial activity of eleven Piper species from distinct rainforest areas in Southeastern Brazil. Ind Crop Prod 94:528–539
Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M (2012) Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem Biol Drug Des 80(3):434–439
Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J (2013) Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem Toxicol 56:352–362
Rajeswari G, Murugan M, Mohan VR (2012) GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae) R J Pharma , Biol Chem Sci 3(4):301–308
Al-Marzoqi AH, Hameed IH, Idan SA (2015) Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr J Biotechnol 14(40):2812–2830
Javed MR, Salman M, Tariq A, Tawab A, Zahoor MK, Naheed S et al (2022) The antibacterial and larvicidal potential of bis-(2-ethylhexyl) phthalate from Lactiplantibacillus plantarum. Molecules 27(21):7220
Tyagi T, Agarwal M (2017) Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J Pharmacog Phytochem 6(1):195–206
Lou-Bonafonte JM, Martínez-Beamonte R, Sanclemente T, Surra JC, Herrera-Marcos LV, Sanchez-Marco J et al (2018) Current insights into the biological action of squalene. Mol Nutr Food Res 62(15):1800136
Saleem M (2009) Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285(2):109–115
Deveci O, Sukan A, Tuzun N, Hames EE (2010) Chemical composition, repellent and antimicrobial activity of Schinus molle L. J Med Plant Res 4(21):2211–2216
Martins MR, Arantes S, Candeias F, Maria Teresa Tinoco MT, Cruz-Morais J (2014) Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J Ethnopharmacol 151:485–492
Ben Hmed M, Rigane G, Ben SR, Zouarl N, Cherif S (2020) Phytochemical and antioxidant activities of Schinus molle L. extract. Rev Roum Chim 65(2):173–178
Pawlowski Â, Kaltchuk-Santos E, Brasil MC, Caramão EB, Zini CA, Soares GLG (2013) Chemical composition of Schinus lentiscifolius March. essential oil and its phytotoxic and cytotoxic effects on lettuce and onion. S Afr J Bot [Internet] 88:198–203. https://doi.org/10.1016/j.sajb.2013.07.026
Inamdar S, Joshi S, Joshi S (2020) Cytotoxicity potential of different extracts of Ziziphus mauritiana (Lam) leaf using Allium cepa model. J Pharmacog Phytochem 9(2):168–172
Santos CA, Silva JR, Silva MR (2012) Cytotoxic and genotoxic effects of Inula viscosa leaf extracts on the root meristem cells of Allium cepa. Caryologia 65(2):97–103
Ramos GQ, Cotta EA, da Fonseca Filho HD (2016) Studies on the ultrastructure in Anacardium occidentale L. leaves from Amazon in northern Brazil by scanning microscopy. Scanning [Internet] 38(4):329–335. https://doi.org/10.1002/sca.21274
Nefic H, Musanovic J, Metovic A, Kurteshi K (2013) Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by alprazolam. Med Arch [Internet] 67(6):388. https://doi.org/10.5455/medarh.2013.67.388-392
Garzoli S, Masci VL, Ovidi E, Turchetti G, Zago D, Tiezzi A (2019) Chemical investigation of a biologically active Schinus molle L. leaf extract. J Anal Methods Chem [Internet] 2019:8391263. https://doi.org/10.1155/2019/8391263
Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater [Internet] 190(1–3):613–621. https://doi.org/10.1016/j.jhazmat.2011.03.095
Ghosh M, Jana A, Sinha S, Jothiramajayam M, Nag A, Chakraborty A et al (2016) Effects of ZnO nanoparticles in plants: cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat Res Genet Toxicol Environ Mutagen [Internet] 807:25–32. https://doi.org/10.1016/j.mrgentox.2016.07.006
Sun Z, Xiong T, Zhang T, Wang N, Chen D, Li S (2019) Influences of zinc oxide nanoparticles on Allium cepa root cells and the primary cause of phytotoxicity. Ecotoxicology [Internet] 28(2):175–188. https://doi.org/10.1007/s10646-018-2010-9
Sudhakar R, Gowda KNN, Venu G (2001) Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia (Tokyo) [Internet] 66(3):235–239. https://doi.org/10.1508/cytologia.66.235
Nwakanma NMC, Okoli BE (2010) Cytological effects of the root extracts of Boerhaavia diffusa on root tips of Crinum jagus. Eurasian J Biosci [Internet]:105–111. https://doi.org/10.5053/ejobios.2010.4.0.13
de Lima M, Nogueira CNA, Santos SC, do, Beijo LA, Barbosa S. (2017) Allelopathic effects of aqueous and ethanolic leaves extracts of Schinus molle L. under different kinds of pruning. J Agric Sci Technol A [Internet] 7(3). https://doi.org/10.17265/2161-6256/2017.03.004
Mahakhode RH, Somkuwar SR (2013) Mitotic abnormalities induced by glyphosate in Psoralea corylifolia L. Int J Curr Pharm Res 5:46–48
Kalsbeek D, Golsteyn R (2017) G2/M-phase checkpoint adaptation and micronuclei formation as mechanisms that contribute to genomic instability in human cells. Int J Mol Sci [Internet] 18(11):2344. https://doi.org/10.3390/ijms18112344
Eleftheriou EP, Adamakis I-DS, Melissa P (2012) Effects of hexavalent chromium on microtubule organization, ER distribution and callose deposition in root tip cells of Allium cepa L. Protoplasma [Internet] 249(2):401–416. https://doi.org/10.1007/s00709-011-0292-3
Ezzat M, Elghamery A, Mahgoub HAM, Shaban AS (2022) Phytotoxicity and genotoxicity evaluations of chromium hexavalent (CrVI) on Allium cepa and Nigella sativa root cells. Egypt J Bot [Internet]. https://doi.org/10.21608/ejbo.2022.164124.2144
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.S. conceptualization, examination, original draft writing; G.M.S. conceptualization, investigation, original draft writing, supervision; M.H.K. writing—original draft preparation, investigation, and supervision; M.S.H. conceptualization, writing—original draft preparation, and investigation. A.S.S. investigation, writing evaluation, and editing. All authors have read and approved the version of the manuscript that has been published.
Corresponding author
Ethics declarations
Ethics approval
This study did not require ethical approval or ethical approval not applicable.
Consent to participate
Written informed consent forms were collected once all study participants received information about it.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
M. E., S., El-Sherbiny, G.M., Sharaf, M.H. et al. Phytochemical analysis, antimicrobial, antioxidant, and cytotoxicity activities of Schinus molle (L.) extracts. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05301-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05301-1