Abstract
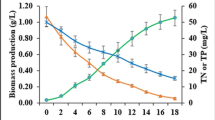
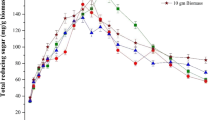
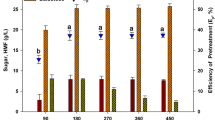
High carbohydrate content of seaweeds (SWs) biomass makes them a potential feedstock for bioethanol production. Improvement of the conversion efficiency from polysaccharide to fermentable sugar during the pretreatment process is helpful to enhance the bioethanol yield. In this study, the response surface methodology (RSM) was used to optimize the thermal-acid pretreatment of SWs (S. thunbergii GEEL-15 and Ulva sp. GEEL-17). Biomass loading, acid concentration, and pretreatment time were studied as independent variables and total reducing sugar (TRS) content was selected as response value. The results showed that the correlation between independent variables with TRS content was significant (p<0.001). The optimal biomass loading for GEEL-15 and GEEL-17 was 9.0% w/v and 5.5% w/v at 85 and 82 min, respectively, at an acid concentration of 1.2% v/v. The TRS content increased upto nearly 150.621 and 262.543 mg/g biomass for GEEL-15 and GEEL-17, respectively.
Graphical abstract






Similar content being viewed by others
Data Availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
Kamaraj A, Ramalingam K (2021) Effect of Cobalt Chromite on the investigation of traditional CI engine powered with raw citronella fuel for the future sustainable renewable source. SAE Int J Adv & Curr Prac in Mobility 3(2):843–850. https://doi.org/10.4271/2020-28-0445
Venugopal IP et al (2023) Quantification of φ-operating range with impact of exhaust gas recirculation under low-temperature combustion mode with polyoxymethylene dimethyl ether: a perspective study. J Clean Prod 411:137298. https://doi.org/10.1016/j.jclepro.2023.137298
Perumal Venkatesan E et al (2021) Effect of Hybrid Nanoparticle on DI Diesel Engine Performance, Combustion, and Emission Studies, in Novel Internal Combustion Engine Technologies for Performance Improvement and Emission Reduction, A.P. Singh and A.K. Agarwal, Editors. Springer Singapore: Singapore. p. 235–263. https://doi.org/10.1007/978-981-16-1582-5_10
Wan Y, Chen Y, Li K (2022) Identification and spatiotemporal distribution analysis of global biomass burning based on Suomi-NPP VIIRS Nightfire data. J Clean Prod 359:131959. https://doi.org/10.1016/j.jclepro.2022.131959
Saravanan K et al (2018) Evaluation of the saccharification and fermentation process of two different seaweeds for an ecofriendly bioethanol production. Biocatal Agric Biotechnol 14:444–449. https://doi.org/10.1016/j.bcab.2018.03.017
El Harchi M, Fakihi Kachkach FZ, El N, Mtili (2018) Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen Tannophilus. S Afr J Bot 115:161–169. https://doi.org/10.1016/j.sajb.2018.01.021
Li M et al (2019) Combustion behavior and oscillatory regime of flame spread over ethanol aqueous solution with different proportions. Fuel 253:220–228. https://doi.org/10.1016/j.fuel.2019.05.018
Jeyakumar N et al (2023) Using Pithecellobium Dulce seed-derived biodiesel combined with Groundnut shell nanoparticles for diesel engines as a well-advised approach toward sustainable waste-to-energy management. Fuel 337:127164. https://doi.org/10.1016/j.fuel.2022.127164
Ağbulut Ü et al (2023) Microalgae bio-oil production by pyrolysis and hydrothermal liquefaction: mechanism and characteristics. Bioresour Technol 376:128860. https://doi.org/10.1016/j.biortech.2023.128860
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340. https://doi.org/10.1016/j.rser.2017.05.225
Hoang AT et al (2023) Biofuel production from microalgae: challenges and chances. Phytochem Rev 22:1089–1126. https://doi.org/10.1007/s11101-022-09819-y
Wang X et al (2020) Economically important red algae resources along the Chinese coast: history, status, and prospects for their utilization. Algal Res 46:101817. https://doi.org/10.1016/j.algal.2020.101817
Wehr JD, Sheath RG, Kociolek JP (2015) Chap. 19 - Brown Algae, in Freshwater Algae of North America (Second Edition), Academic Press: Boston. p. 851–871
Yuan Y et al (2018) Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: functional properties and bioactivities. Carbohydr Polym 181:902–910. https://doi.org/10.1016/j.carbpol.2017.11.061
Trivedi N et al (2013) Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour Technol 150:106–112. https://doi.org/10.1016/j.biortech.2013.09.103
Trivedi N et al (2015) Solid state fermentation (SSF)-derived cellulase for saccharification of the green seaweed Ulva for bioethanol production. Algal Res 9:48–54. https://doi.org/10.1016/j.algal.2015.02.025
Polikovsky M et al (2020) Engineering bacteria-seaweed symbioses for modulating the photosynthate content of Ulva (Chlorophyta): significant for the feedstock of bioethanol production. Algal Res 49:101945. https://doi.org/10.1016/j.algal.2020.101945
Li Y et al (2016) Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour Technol 214:144–149. https://doi.org/10.1016/j.biortech.2016.04.090
Qarri A, Israel A (2020) Seasonal biomass production, fermentable saccharification and potential ethanol yields in the marine macroalga Ulva sp. (Chlorophyta). Renew Energ 145:2101–2107. https://doi.org/10.1016/j.renene.2019.07.155
Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgae Sargassum spp. Bioresour Technol 138:22–29. https://doi.org/10.1016/j.biortech.2013.03.108
Veza I et al (2022) Microalgae and Macroalgae for Third-Generation Bioethanol Production, in Liquid Biofuels. In: Bioethanol CR, Soccol et al (eds) Editors. Springer International Publishing, Cham, pp 301–331
Hong IK, Jeon H, Lee SB (2014) Comparison of red, brown and green seaweeds on enzymatic saccharification process. J Ind Eng Chem 20:2687–2691. https://doi.org/10.1016/j.jiec.2013.10.056
Lakshmikandan M et al (2021) Optimization of acid hydrolysis on the green seaweed Valoniopsis pachynema and approach towards mixotrophic microalgal biomass and lipid production. Renew Energ 164:1052–1061. https://doi.org/10.1016/j.renene.2020.10.062
Tan IS, Lee KT (2014) Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: an optimization study. Energy 78:53–62. https://doi.org/10.1016/j.energy.2014.04.080
Eren Şenaras A (2019) Chap. 8 - Parameter optimization using the surface response technique in automated guided vehicles, in Sustainable Engineering Products and Manufacturing Technologies, K. Kumar, D. Zindani and P. Davim, Editors, Academic Press. p. 187–197
Sharma M et al (2023) Evaluation of aerobic biodegradation of phenanthrene using Pseudomonas turukhanskensis: an optimized study. Biodegradation 34:21–41. https://doi.org/10.1007/s10532-022-10002-5
Bakshi A, Verma AK, Dash AK (2020) Electrocoagulation for removal of phosphate from aqueous solution: statistical modeling and techno-economic study. J Clean Prod 246:118988. https://doi.org/10.1016/j.jclepro.2019.118988
Kumari M, Gupta SK (2019) Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modified magnetic nanoadsorbents (sMNP) - an endeavor to diminish probable cancer risk. Sci Rep 9:18339. https://doi.org/10.1038/s41598-019-54902-8
Pandya DK, Kumar MA (2021) Chemo-metric engineering designs for deciphering the biodegradation of polycyclic aromatic hydrocarbons. J Hazard Mater 411:125154. https://doi.org/10.1016/j.jhazmat.2021.125154
Yang Y et al (2022) Potential of Marine seaweeds for Bioactive compounds: a comprehensive analysis of Padina Australis Biomass. Thalassas: An International Journal of Marine Sciences 38:947–956. https://doi.org/10.1007/s41208-022-00436-2
Yang Y et al (2021) Identification and characterization of marine seaweeds for biocompounds production. Environ Technol Innov 24. https://doi.org/10.1016/j.eti.2021.101848
Manirakiza P, Covaci A, Schepens P (2001) Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J Food Compost Anal 14:93–100. https://doi.org/10.1006/jfca.2000.0972
Waterborg JH (2009) The Lowry Method for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Humana Press, Totowa, NJ, pp 7–10
Breil C et al (2017) Bligh and Dyer and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of Solvatation mechanisms and towards substitution with alternative solvents. Int J Mol Sci 18. https://doi.org/10.3390/ijms18040708
Nielsen SS (2010) Phenol-sulfuric acid method for total carbohydrates. In: Nielsen SS (ed) Food Analysis Laboratory Manual. Springer US, Boston, MA, pp 47–53
Miller GL (1959) Use of Dinitrosalicylic Acid Reagent for determination of reducing Sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Hu R et al (2008) Reducing sugar content in hemicellulose hydrolysate by DNS method: a revisit. J Biobased Mater Bio 2:156–161. https://doi.org/10.1166/jbmb.2008.306
Mahmoodi-Babolan N, Heydari A, Nematollahzadeh A (2019) Removal of methylene blue via bioinspired catecholamine/starch superadsorbent and the efficiency prediction by response surface methodology and artificial neural network-particle swarm optimization. Bioresour Technol 294:122084. https://doi.org/10.1016/j.biortech.2019.122084
Mattsson K et al (2015) Altered behavior, physiology, and metabolism in Fish exposed to Polystyrene nanoparticles. Environ Sci Technol 49:553–561. https://doi.org/10.1021/es5053655
Dixit S, Yadav VL (2019) Optimization of polyethylene/polypropylene/alkali modified wheat straw composites for packaging application using RSM. J Clean Prod 240. https://doi.org/10.1016/j.jclepro.2019.118228
Kamal S et al (2022) Sustainable and optimized bioethanol production using mix microbial consortium of Saccharomyces cerevisiae and Candida Cantarelli. Fuel 314:122763. https://doi.org/10.1016/j.fuel.2021.122763
Manimaran DR et al (2021) Response surface analysis and optimization of inulin extraction from garlic. Biomass Convers Bior 13:10845–10851. https://doi.org/10.1007/s13399-021-01953-5
Panjiara D, Pramanik H (2020) Optimization of process parameters using response surface methodology (RSM) for power generation via electrooxidation of glycerol in T-Shaped air breathing microfluidic fuel cell (MFC). Int J Hydrog Energy 45:33968–33979. https://doi.org/10.1016/j.ijhydene.2020.09.023
Zulkali MMD, Ahmad AL, Norulakmal NH (2006) Oryza sativa L. husk as heavy metal adsorbent: optimization with lead as model solution. Bioresour Technol 97:21–25. https://doi.org/10.1016/j.biortech.2005.02.007
Mohammed A et al (2020) Alginate extraction from Sargassum seaweed in the Caribbean region: optimization using response surface methodology. Carbohyd Polym 245:116419. https://doi.org/10.1016/j.carbpol.2020.116419
Rodrigues AC et al (2019) Response surface statistical optimization of bacterial nanocellulose fermentation in static culture using a low-cost medium. N Biotechnol 49:19–27. https://doi.org/10.1016/j.nbt.2018.12.002
Tantayotai P et al (2022) In-depth investigation of the bioethanol and biogas production from organic and mineral acid pretreated sugarcane bagasse: comparative and optimization studies. Biocatal Agric Biotechnol 45:102499. https://doi.org/10.1016/j.bcab.2022.102499
Ruangrit K et al (2021) Environmental-friendly pretreatment and process optimization of macroalgal biomass for effective ethanol production as an alternative fuel using Saccharomyces cerevisiae. Biocatal Agric Biotechnol 31. https://doi.org/10.1016/j.bcab.2021.101919
He M et al (2023) Total flavonoids in Artemisia absinthium L. and evaluation of its anticancer activity. https://doi.org/10.21203/rs.3.rs-3032507/v1
Kim N-J et al (2011) Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour Technol 102:7466–7469. https://doi.org/10.1016/j.biortech.2011.04.071
Funding
The authors are thankful to Deanship of Scientific Research and under the supervision of the Scientific and Engineering Research Center at Najran University for funding this work under the Research centers Funding program grant code (NU/RCP/SERC/12/11).
Author information
Authors and Affiliations
Contributions
Yulu Yang: Methodology, Investigation, Visualization, Formal analysis, Writing-original draft preparation, Writing-review and editing, Revision. Mohammed Jalalah: Data curation, Funding support, Writing-review, and editing. Saeed A. Alsareii: Methodology, Investigation, Formal analysis. Farid A. Harraz: Methodology, Formal analysis. Abdulrhman A. Almadiy: Methodology, Formal analysis. Nandini Thakur: Methodology, Formal analysis, Writing-original draft preparation. El-Sayed Salama: Supervision, Resources, Data curation, Validation, Visualization, Formal analysis, Writing-review and editing, Funding acquisition, Project administration, Revision.
Corresponding authors
Ethics declarations
Institutional Review Board Statement
Not applicable.
Informed consent
Not applicable.
Ethics approval
This article does not contain any studies with human participants or animals performed by any authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Jalalah, M., Alsareii, S.A. et al. Enhancement of total reducing sugar content from seaweeds (SWs) biomass via pretreatment for ethanol production: an optimized study. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-05186-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-05186-6