Abstract
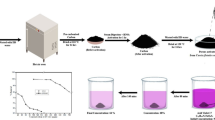
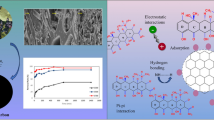
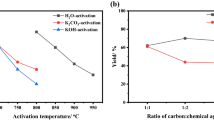
In this study, adsorption, a promising technique due to its simplicity and high efficiency, was used to remove tetracycline and ciprofloxacin from water. One-step activated carbon production from lignocellulosic waste uses an environmentally friendly, effective, economical, and sustainable adsorbent. The study aimed to remove TC and CP from an aqueous solution. The effect of parameters such as pH (3–11), biosorbent dose (0.25–5 g/L), initial antibiotic concentration (10–150 mg/L), and contact time (5–240 min) on adsorption was investigated. It was observed that the adsorption process was relatively fast, and equilibrium was reached within 120 and 90 min for tetracycline and ciprofloxacin, respectively. Furthermore, it showed the best correlation with the Langmuir isotherm model. The theoretical capacities of crude activated carbon waste to adsorb tetracycline and ciprofloxacin were determined as 29.16 and 46.37 mg/g, respectively. The experimental results obtained in this study indicate that this agricultural waste can be used as an environmentally friendly and cost-effective adsorbent to remove some classes of antibiotics from aqueous solutions, thus contributing to the solution of the problem of the uncontrolled presence of these drugs in wastewater.
Graphical Abstract












Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Zhang D et al (2014) Removal of pharmaceuticals and personal care products in aquatic plant-based systems: a review. Environ Pollut 184:620–639
Debnath B et al (2020) The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J Environ Manage 261:110235
Zhang M et al (2019) Fate of veterinary antibiotics during animal manure composting. Sci Total Environ 650:1363–1370
Conde-Cid M et al (2020) Adsorption/desorption of three tetracycline antibiotics on different soils in binary competitive systems. J Environ Manage 262:110337
Charuaud L et al (2019) Veterinary pharmaceutical residues from natural water to tap water: sales, occurrence and fate. J Hazard Mater 361:169–186
Mosaleheh N, Sarvi MN (2020) Minimizing the residual antimicrobial activity of tetracycline after adsorption into the montmorillonite: effect of organic modification. Environ Res 182:109056
Zhao Y et al (2011) Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J Colloid Interface Sci 361(1):247–251
Zhao Y et al (2012) Adsorption of tetracycline (TC) onto montmorillonite: cations and humic acid effects. Geoderma 183:12–18
Ferreira VR et al (2016) Fluoroquinolones biosorption onto microbial biomass: activated sludge and aerobic granular sludge. Int Biodeter Biodegr 110:53–60
Xia C et al (2023) An effective strategy for removing tetracycline from water: enhanced adsorption reliability and capacity by tyrosine modified layered hydroxides. J Environ Chem Eng 11(1):109172
Gaballah MS et al (2021) A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour Technol 333:125069
Sullivan BA et al (2017) Effects of chlorination and ultraviolet light on environmental tetracycline-resistant bacteria and tet (W) in water. J Environ Chem Eng 5(1):777–784
Organization, WH (2016) World health statistics 2016: monitoring health for the SDGs sustainable development goals. World Health Organization
Yu F et al (2022) Batch and continuous fixed-bed column adsorption of tetracycline by biochar/MOFs derivative covered with κ-carrageenan/calcium alginate hydrogels. J Environ Chem Eng 10(3):107996
Dehmani Y et al (2022) Adsorption removal of phenol by oak wood charcoal activated carbon. Biomass Convers Biorefin:1–13
Yazidi A et al (2020) Adsorption of amoxicillin and tetracycline on activated carbon prepared from durian shell in single and binary systems: experimental study and modeling analysis. Chem Eng J 379:122320
Mokhtari P et al (2016) Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: kinetic and isotherm investigation. J Mol Liq 219:299–305
Affam AC et al (2018) Preparation, characterization and adsorption study of granular activated carbon/iron oxide composite for the removal of boron and organics from wastewater. In: E3S Web of Conferences. EDP Sciences.
Movasaghi Z, Yan B, Niu C (2019) Adsorption of ciprofloxacin from water by pretreated oat hulls: equilibrium, kinetic, and thermodynamic studies. Ind Crop Prod 127:237–250
Shao S et al (2019) Biodegradation mechanism of tetracycline (TEC) by strain Klebsiella sp. SQY5 as revealed through products analysis and genomics. Ecotoxicol Environ Saf 185:109676
Yi K et al (2017) Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci Total Environ 605:368–375
Xu Q et al (2020) Effect of extracellular polymer substances on the tetracycline removal during coagulation process. Bioresour Technol 309:123316
Parsa JB, Panah TM, Chianeh FN (2016) Removal of ciprofloxacin from aqueous solution by a continuous flow electro-coagulation process. Korean J Chem Eng 33(3):893–901
Saitoh T, Shibata K, Hiraide M (2014) Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation–sedimentation method. J Environ Chem Eng 2(3):1852–1858
Kitazono Y et al (2012) Selective degradation of tetracycline antibiotics present in raw milk by electrochemical method. J Hazard Mater 243:112–116
Palacio DA et al (2020) Tetracycline removal by polyelectrolyte copolymers in conjunction with ultrafiltration membranes through liquid-phase polymer-based retention. Environ Res 182:109014
Qalyoubi L, Al-Othman A, Al-Asheh S (2022) Removal of ciprofloxacin antibiotic pollutants from wastewater using nano-composite adsorptive membranes. Environ Res 215:114182
Peng Y et al (2021) Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: a review. Chem Eng J 414:128800
Mondal SK, Saha AK, Sinha A (2018) Removal of ciprofloxacin using modified advanced oxidation processes: kinetics, pathways and process optimization. J Clean Prod 171:1203–1214
Acero JL et al (2010) Kinetics of aqueous chlorination of some pharmaceuticals and their elimination from water matrices. Water Res 44(14):4158–4170
Wang H et al (2017) Interaction of ciprofloxacin chlorination products with bacteria in drinking water distribution systems. J Hazard Mater 339:174–181
Avcı A, İnci İ, Baylan N (2020) Adsorption of ciprofloxacin hydrochloride on multiwall carbon nanotube. J Mol Struct 1206:127711
Sayğılı H, Güzel F (2016) Effective removal of tetracycline from aqueous solution using activated carbon prepared from tomato (Lycopersicon esculentum Mill.) industrial processing waste. Ecotoxicol Environ Saf 131:22–29
Marzbali MH et al (2016) Tetracycline adsorption by H3PO4-activated carbon produced from apricot nut shells: a batch study. Process Saf Environ Prot 102:700–709
Huang L et al (2014) Adsorption of tetracycline and ciprofloxacin on activated carbon prepared from lignin with H3PO4 activation. Desalin Water Treat 52(13-15):2678–2687
Zhou J, Ma F, Guo H (2020) Adsorption behavior of tetracycline from aqueous solution on ferroferric oxide nanoparticles assisted powdered activated carbon. Chem Eng J 384:123290
Liang J et al (2019) Magnetic nanoferromanganese oxides modified biochar derived from pine sawdust for adsorption of tetracycline hydrochloride. Environ Sci Pollut Res 26:5892–5903
Ahamad T et al (2020) Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: kinetic and thermodynamic studies. Int J Biol Macromol 147:258–267
Sekulic MT et al (2019) Surface functionalised adsorbent for emerging pharmaceutical removal: adsorption performance and mechanisms. Process Saf Environ Prot 125:50–63
Pi Y et al (2018) Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem Eng J 337:351–371
Arslanoğlu H, Çiftçi H (2021) Use of sulfuric acid-carbonization materials from grape pulp for the removal of hexavalent chromium (Cr (VI)): mechanism and characterization. Int J Phytoremediation 23(11):1145–1156
Arslanoğlu E et al (2021) Fabrication, characterization, and adsorption applications of low-cost hybride activated carbons from peanut shell-vinasse mixtures by one-step pyrolysis. Biomass Convers Biorefin:1–15
Arslanoğlu H (2019) Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J Hazard Mater 374:238–247
Seyrek EŞ et al (2020) Effect of activated carbon obtained from vinasse and marc on the rheological and mechanical characteristics of the bitumen binders and hot mix asphalts. Construct Build Mater 240:117921
Sevilla M, Fuertes AB (2013) A general and facile synthesis strategy towards highly porous carbons: carbonization of organic salts. J Mater Chem A 1(44):13738–13741
Turku I, Sainio T, Paatero E (2007) Thermodynamics of tetracycline adsorption on silica. Environ Chem Lett 5:225–228
Yao J et al (2023) Role of magnetic substances in adsorption removal of ciprofloxacin by gamma ferric oxide and ferrites co-modified carbon nanotubes. J Colloid Interface Sci 638:872–881
Kumar A et al (2022) Effect of magnetization on the adsorptive removal of an emerging contaminant ciprofloxacin by magnetic acid activated carbon. Environ Res 206:112604
Zhang Y et al (2018) Removal of tetracycline by coupling of flow-through electro-Fenton and in-situ regenerative active carbon felt adsorption. Chem Eng J 335:685–692
Zhang D et al (2015) Adsorption and removal of tetracycline from water by petroleum coke-derived highly porous activated carbon. J Environ Chem Eng 3(3):1504–1512
Avcı A, İnci İ, Baylan N (2019) A comparative adsorption study with various adsorbents for the removal of ciprofloxacin hydrochloride from water. Water Air Soil Pollut 230:1–9
Ouaissa YA et al (2014) Removal of tetracycline by electrocoagulation: kinetic and isotherm modeling through adsorption. J Environ Chem Eng 2(1):177–184
Arslanoglu H, Altundogan HS, Tumen F (2009) Heavy metals binding properties of esterified lemon. J Hazard Mater 164(2-3):1406–1413
Gao Y et al (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368(1):540–546
Al-Ghouti MA, Da'ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: a review. J Hazard Mater 393:122383
Kong Q et al (2016) Isotherm, kinetic, and thermodynamic equations for cefalexin removal from liquids using activated carbon synthesized from loofah sponge. Desalin Water Treat 57(17):7933–7942
Ward TJ, Farris Iii AB (2001) Chiral separations using the macrocyclic antibiotics: a review. J Chromatogr A 906(1-2):73–89
Wang S, Wang H (2015) Adsorption behavior of antibiotic in soil environment: a critical review. Front Environ Sci Eng 9:565–574
Oberoi AS et al (2019) Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol 53(13):7234–7264
Acknowledgements
Firat University and Izmir High Technology University are thanked in this article for their analysis, testing, and laboratory facilities.
Author information
Authors and Affiliations
Contributions
Buket Onat: writing, revising the manuscript critically for important intellectual content. Feride N. Türk: analysis of data, revising the manuscript critically for important intellectual content, approval of the version of the manuscript to be published. Hasan Arslanoğlu: writing, acquisition of data, analysis of data, revising the manuscript critically for important intellectual content, approval of the version of the manuscript to be published, review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Adsorption of tetracycline and ciprofloxacin from aqueous solution by activated carbon obtained from agricultural waste was investigated.
• The highest surface area and total pore volume of the produced activated carbon based adsorbent were found to be 1290.5 m2/g and 0.5667 cm3/g, respectively.
• Tetracycline (TC) showed cationic property at high pH and the highest adsorption capacity were observed at pH:10.
• Ciprofloxacin (CIP) showed anionic property at low pH and the highest adsorption capacity were observed at pH:4.
• The adsorbent corresponds to Type 4 in adsorption isotherm and consists mostly of microporous.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Onat, B., Türk, F.N. & Arslanoğlu, H. Synthesis of high porous carbon from grape marc-vinasse mixture: investigation on tetracycline and ciprofloxacin removal performance and adsorption mechanisms. Biomass Conv. Bioref. 14, 10733–10745 (2024). https://doi.org/10.1007/s13399-023-04896-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04896-1