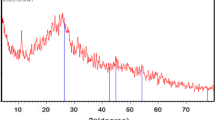
Abstract
The present study deals with the preparation and characterization of porous activated carbon from Cassia fistula seed shell (CFSS), an agriculture solid waste (byproducts) for the removal synthetic anionic Acid Violet (AV17) dye by batch adsorption technique, and the results were compared with commercial activated carbon (CAC). BET surface area and point zero charge of the CFSSC is 485.5 m2/g and 6.5. The as prepared CFSSC structural and morphological features were confirmed using XRD, FITR, TGA, and SEM analysis. The effect of diverse experimental parameters such as dosage of adsorbent, contact time, initial concentration of AV17 dye, and pH on the removal of dye by adsorption using the adsorbents (CFSSC and CAC) has been studied in room temperature. The Langmuir and Freundlich isotherm models were applied. The adsorption capacities of the CAC and CFSSC on AV17 dye was found to be 290.90 and 132.45 mg/g, respectively. The adsorption kinetics initiates to be first order with respect to intra-particle diffusion as rate determining step. The process of removal of dyes by adsorption on CAC and CFSSC was found to be highly pH dependent. The maximum adsorption observed at pH 2. The result in the present study indicates that CFSSC could be used as a cost-effective alternative adsorbent for the removal of dilute acidic dyes from wastewater instead of CAC.













Similar content being viewed by others
Data availability
This is not applicable.
References
Soumi D, Bramha G, Suneel KS, Ashok KG (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2:4497–4531
Muhammad B, Ihsanullah I, Mansoor Ul HS, Ambavaram VBR, Tejraj MA (2022) Recent advances in the removal of dyes from wastewater using low-cost adsorbents. J Environ Manag 21:115981
Anastopoulos I, Bhatnagar A, Hameed BH, Ok YS, Omirou M (2020) A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J Mol Liq 240:179–188
Bhatnagar A, Anastopoulos I (2017) Adsorptive removal of bisphenol A (BPA) from aqueous solution: a review. Chemosphere 168:885–902
Aragaw TA, Bogale FM (2021) Biomass-based adsorbents for removal of dyes from wastewater: a review. Front Environ Sci 9:764958
Bhatnagar A, Sillanpaa M (2010) Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment - a review. Chem Eng J 157:277–296
Sivakumar S, Muthirulan P, Meenakshi SM (2019) Adsorption kinetic and isotherm studies of Azure A on various activated carbons derived from agricultural wastes. Arab J Chem 12:1507–1514
Divine Angela GS, Sergio CC, Mark Daniel GL (2018) Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes. J. Environ Manag 210:255–262
Sonalika S, Prem P, Brijesh KM, Nayak GC (2020) Synthesis, characterization and sorption studies of a zirconium(IV) impregnated highly functionalized mesoporous activated carbons. RSC Adv 10:13783–13798
Kannan N, Pagutharivalan R (2012) Removal of Basic Green dye from aqueous media by using Eucalyptus globules bark carbon as an adsorbent-a comparative study. J Chem Pharm Res 4:38–45
Shahul Hameed K, Muthirulan P, Meenakshi SM (2017) Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: equilibrium and kinetics studies. Arab J Chem 10:S2225–S2233
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Kannan N, Meenakshi SM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons – a comparative study. Dyes Pigm 51:25–40
Langmuir I (1918) The adsorption of gases on plane surfaces of glass mica and platinum. J Am Chem Soc 40:1361–1403
Uttara M, Ajay Kumar M, Abhijit C (2022) A critical evaluation of conventional kinetic and isotherm modeling for adsorptive removal of hexavalent chromium and methylene blue by natural rubber sludge-derived activated carbon and commercial activated carbon. Bioresour Technol 343:126135–126146
McKay CR (1983) The adsorption of dyestuff from aqueous solution using activated carbon analytical solution for batch adsorption based on external mass transfer and pore diffusion. Chem Eng J 27:187
Nasuha N, Hameed BH (2011) Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem Eng J 166:783–786
Madrakian T, Afkhami A, Ahmadi M (2012) Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim Acta Part A Mol Biomol Spectrosc 99:102–109
Jain SN, Gogate PR (2017) Adsorptive removal of acid violet 17 dye from wastewater using biosorbent obtained from NaOH and H2SO4 activation of fallen leaves of Ficus Racemosa. J Mol Liq 243:132–143
Garg D, Kumar S, Sharma K, Majumder CB (2019) Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw Sustain Dev 8:512–519
Cretescu I, Lupascu T, Buciscanu I, Balau-Mindru T, Soreanu G (2017) Low-cost sorbents for the removal of acid dyes from aqueous solutions. Process Saf Environ Protect 108:57–66
Guerrero-Coronilla I, Morales-Barrera L, Cristiani-Urbina E (2015) Kinetic, isotherm and thermodynamic studies of amaranth dye biosorption from aqueous solution onto water hyacinth leaves. J Environ Manag 152:99–108
Bhomick PC, Supong A, Baruah M, Pongener C, Sinha D (2018) Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: isotherm, kinetic, thermodynamic and regeneration studies. Sustain Chem Pharm 10:41–49
Khan S, Malik A (2018) Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ Sci Pollut Res 25:4446–4458
Shindhal T, Rakholiya P, Varjani S, Pandey A, Ngo HH, Guo W, Ng HY, Taherzadeh MJ (2021) A critical review on advances in the practices and perspectives for the treatment of dye industry waste water. Bioengineered 12:70–87
Jamee R, Siddique R (2019) Biodegradation of synthetic dyes of textile effluent by microorganisms: an environmentally and economically sustainable approach. Eur J Microbiol Immunol 9:114–118
Khattab TA, Abdelrahman MS, Rehan M (2020) Textile dyeing industry: environmental impacts and remediation. Environ Sci Pollut Res 27:3803–3818
Hameed BH, Din ATM, Ahmad AL (2007) Adsorption of methylene blue onto bamboo- based activated carbon: kinetics and equilibrium studies. J Hazard Mater 141:819–825
Lianggui W (2012) Application of activated carbon derived from ‘waste’ bamboo culms for the adsorption of azo disperse dye: kinetic, equilibrium and thermodynamic studies. J Environ Manag 102:79–87
Hem L, Garg VK, Gupta RK (2008) Adsorptive removal of basic dye by chemically activated Parthenium biomass: equilibrium and kinetic modeling. Desalination 219:250–261
Kavitha D, Namsivayam C (2002) Capacity of activated carbon in the removal of acid brilliant blue: determination of equilibrium and kinetic model parameters. Chem Eng J 139:453–461
Robinson T, Chandran B, Nigam P (2002) Effect of pretreatments of three waste residues, wheat straw, corncobs and barley husks on dye adsorption. Bioresour Technol 85:119–124
Jayarajan M, Arunachalam R, Annadurai G (2011) Agricultural wastes of jackfruit peel nano-porous adsorbent for removal of rhodamine dye. Asian J Appl Sci 4:263–270
Jain SN, Gogate PR (2018) Efficient removal of Acid Green 25 dye from wastewater using activated Prunus Dulcis as biosorbent: Batch and column studies. J Environ Manage 210:226–238
Thinakaran N, Baskaralingam P, Pulikesi M, Panneerselvamc P, Sivanesan S (2008) Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J Hazard Mater 151:316–322
Kannan N, Murugavel S (2008) Comparative study on the removal of Acid Violet by adsorption on various low cost adsorbents. Glob Nest J 10:395–403
Anjaneya O, Santoshkumar M, Nayak Anand S, Karegoudar TB (2009) Biosorption of acid violet dye from aqueous solutions using native biomass of a new isolate of Penicillium species. Int Biodeterior Biodegrad 63:782–787
Sivaraj R, Namasivayam C, Kadirvelu K (2001) Orange peel as an adsorbent in the removal of Acid violet 17 (acid dye) from aqueous solutions. Waste Manage 21:105–110
Vijayalakshmi P, Bala VSS, Thiruvengadaravi KV, Panneerselvam P, Palanichamy M, Sivanesan S (2011) Removal of Acid Violet 17 from aqueous solutionsby adsorption onto activated carbon prepared from pistachio nut shell. Sep Sci Technol 46:155–163
Thinakaran N, Baskaralingam P, Pulikesi M, Panneerselvam P, Sivanesan S (2008) Removal of Acid Violet 17 from aqueous solutions by adsorption onto activated carbon prepared from sunflower seed hull. J Hazard Mater 151:316–322
Acknowledgments
The authors gratefully thank The Principal and Departments of Chemistry of Rani Anna Government College for Women and The Management and Principal of Lekshmipuram College of Arts and Science, both affiliated with Manonmaniam Sundaranar University in Tirunelveli, India, for providing the research facilities for this experimental investigation.
Author information
Authors and Affiliations
Contributions
Murugan Thavasikannu: conceptualization, methodology, experiments and data analysis, and manuscript writing and editing. Archana Shanmugam: conceptualization, methodology, and data analysis. Rajeswaran Ramaraj: manuscript writing and editing. Banumathi Nagarathinam: methodology, proofreading, and editing. Shylasree GV: resources and methodology. Muthirulan Pandi: funding acquisition, resources, supervision, proofreading, and editing.
Corresponding author
Ethics declarations
Ethical approval
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramaraj, R., Shanmugam, A., Nagarathinam, B. et al. Agriculture byproduct-derived versatile Cassia fistula seed shell carbon for the removal of acid violet 17 dye from aqueous solution: adsorption kinetics, equilibrium, and mechanism studies. Biomass Conv. Bioref. 13, 9507–9523 (2023). https://doi.org/10.1007/s13399-023-04148-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04148-2