Abstract
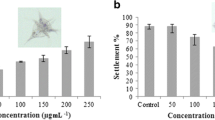
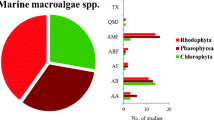
The antifouling potential of crude organic extracts from five seaweed species collected at the Rameswaram coastal region at Pamban station, Tamil Nadu, has been screened for antifouling activity. Five different species of seaweeds such as two Chlorophyceae (Ulva fasciata and Halimeda tuna), one Rhodophyceae (Portieria hornemanni), and two Phaeophyceae (Padina boergesenii and Turbinaria ornata) did not exhibit fouling inhibition. From the marine macroalgae that displayed antifouling activity, P. hornemanni has shown better results when compared to that of other species. The seaweed extracts were tested in laboratory assays against the marine fouling bacteria’s such as B. mycoides, B. fluxes, B. cereus, B. vietnamensis, and P. stutzeri. The GC–MS profile of P. hornemanni suggested the purified fraction is primarily composed of a total of 15 compounds recorded. The maximum byssal thread inhibition of the green mussel Perna viridis was inhibited by the methanol and chloroform extract of P. hornemannii, and the methanol extract of the MIC was found to be 154 8.07 g/ml, followed by EC50 73 ± 3.54 g/ml, respectively. The MIC was determined to be 124 ± 6.54 g/ml in chloroform extract, while the EC50 was determined to be 59 ± 6.74 g/ml. The phytochemical constituents were recorded in methanol extracts except for glycosides and tannins, and the results showed that alkalods, flavnoids, terpenoids, triterpenes, and phenols were found to be 116.6, 72.54, 97.12, 66.34, and 99.78 µg/mg respectively. Which could have a functional role in the chemical defense against marine fouling organisms, and it could be utilized for the improvement of ideal antifoulants in the future.





Similar content being viewed by others
Data availability
Data is available on request from the authors.
References
Sahoo D, Sahu N, Sahoo D (2003) A critical survey of seaweed diversity of Chilika Lake. India Algae 18(1):1–12
Mantri VA, Rao PS (2005) Diu island: a paradise for tourists and seaweed biologists. Current Sci 89(11):1795–1797
Kaliaperumal N (2007) Present status of marine algal biodiversity in Gulf of Manner region. Tamil Nadu Indian Hydro 10(1):53–62
James JE, Kumar RA, Raj AD (2004) Marine algal flora from some localities of southeast coast of Tamil Nadu. Seaweed Res Utiln 26:3–9
Chew YL, Lim YY, Omar M, Khoo KS (2008) Antioxidant activity of three edible seaweeds from two areas in South East Asia LWT-Food. Sci Tech 41(6):1067–1072
Wiencke C, Bischof K (2012) Seaweed biology. Springer-Verlag, Berlin, Heidelberg
Qi SH, Zhang SI, Yang LH, Qian PY (2008) Antifouling and antibacterial compounds from the gorgonians Subergorgia suberosa and Scripearia gracillis. Nat Prod Res 22(2):154–66
Yebra DM, Kiil S, Dam-Johansen K (2004) Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog Org Coat 50(2):75–104
Buskens P, Wouters M, Rentrop C, Vroon Z (2013) A brief review of environmentally benign antifouling and foul-release coatings for marine applications. J Coat Tech Res 10(1):29–36
Malini M, Thirumavalavan M, Yang WY, Lee JF, Annadurai G (2015) A versatile chitosan/ZnO nanocomposite with enhanced antimicrobial properties. Inter J Biol Macromol 1(80):121–129
Maréchal JP, Hellio C (2011) Antifouling activity against barnacle cypris larvae: do target species matter (Amphibalanus amphitrite versus Semibalanus balanoides)? Inter Biodet Biodegrad 65(1):92–101
Koyanagi S, Nakagawa H, Kuramoto Y, Ohdo S, Soeda S, Shimeno H (2003) Optimizing the dosing schedule of TNP-470 [O-(chloroacetyl-carbamoyl) fumagillol] enhances its antitumor and antiangiogenic efficacies. J Pharmacol Exp Therap 304(2):669–74
Bhosle NB, Nandakumar K, Wagh AB (1990) Influence of particulate matter on microfouling biomass in the Arabian Sea. Biofoul 2:65–74
Dhanasekaran D, Thajuddin N, Rashmi M, Deepika TL, Gunasekaran M (2009) Screening of biofouling activity in marine bacterial isolate from ship hull. Inter J Environl Sci Tech 6(2):197–202
Boudjella H, Bouti K, Zitouni A, Mathieu F, Lebrihi A, Sabaou N (2006) Taxonomy and chemical characterization of antibiotics of Streptosporangium Sg 10 isolated from a Saharan soil. Microbiol Res 161(4):288–298
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–25
Felsenstein J (1985) Phylogenies and the comparative method. Amer Natural 125(1):1–15
Moovendhan M, Ramasubburayan R, Vairamani S, Shanmugam A, Palavesam A, Immanuel G (2015) Antibiotic efficacy and characterization of mangrove metabolites against UTI microbes. J Herbs, Spi & Med Plant 21(2):129–139
Hazra B, Biswas S, Mandal N (2008) Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Compl Alter Med 8(1):1
Ghorai N, Chakraborty S, Gucchait S, Saha SK, Biswas S (2012) Estimation of total terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Protocol Exchange. https://doi.org/10.1038/protex.2012.055
Ejikeme C, Ezeonu CS, Eboatu AN (2014) Determination of physical and phytochemical constituents of some tropical timbers indigenous to nigerdelta area of nigeria. Europ Sci J 10(18):247–270
Chaudhuri D, Ghate NB, Sarkar R, Mandal N (2012) Phytochemical analysis and evaluation of antioxidant and free radical scavenging activity of Withania somnifera root. Asian J Pharm Clin Res 5(4):93–199
Chaudhuri D, Ghate NB, Deb S, Panja S, Sarkar R, Rout J, Mandal N (2014) Assessment of the phytochemical constituents and antioxidant activity of a bloom forming microalgae Euglena tuba. Biol Res 47(1):1–1
Moubayed NM, Al Houri HJ, Al Khulaifi MM, Al Farraj DA (2017) Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saud J Biol Sci 24(1):162–169
Ramasubburayan R, Titus S, Kumar P, Immanuel G, Palavesam A (2017) Antifouling activity of marine epibiotic bacterium Bacillus flexus APGI isolated from Kanyakumari Coast. Tamilnadu, India
Adesalu TA, Temenu TO, Julius ML (2016) Molecular characterization, lipid analysis and GC-MS determination of bioactive compounds identified in a West African strain of the green alga Oedogonium (Chlorophyta). J Pharm Phytochem 5(6):1
Venkatesan R, Murthy PS, Vedaprakash L (2006) In marine antfouilng systems a ready reckoner handbook on tīme line, regulations, advances and test protocols. Allied Publishers Private Limited, Chennai
Pati SK, Rao MV (2012) Growth studies of foulers in a polluted Indian harbour. J Marine Biol Asso Ind 54(1):30–37
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ (1987) Bacterial biofilms in nature and disease. Annual Rev Microbiol 41(1):435–464
Ortega-Morales BO, Santiago-García JL, Chan-Bacab MJ, Moppert X, Miranda-Tello E, Fardeau ML, Carrero JC, Bartolo-Pérez P, Valadéz-González A, Guezennec J (2007) Characterization of extracellular polymers synthesized by tropical intertidal biofilm bacteria. J Appl Microbio 102(1):254–264
Lade H, Paul D, Kweon JH (2014) Isolation and molecular characterization of biofouling bacteria and profiling of quorum sensing signal molecules from membrane bioreactor activated sludge. Intern J Mol Sci 15(2):2255–2273
Lim S, Kim S, Yeon KM, Sang BI, Chun J, Lee CH (2012) Correlation between microbial community structure and biofouling in a laboratory scale membrane bioreactor with synthetic wastewater. Desalination 287:209–215
Dang H, Lovell CR (2000) Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl Enviro Microbiol 66(2):467–475
Jones PR, Cottrell MT, Kirchman DL, Dexter SC (2007) Bacterial community structure of biofilms on artificial surfaces in an estuary. Microb Ecol 53(1):153–162
Battin TJ, Sloan WT, Kjelleberg S, Daims H, Head IM, Curtis TP, Eberl L (2007) Microbial landscapes: new paths to biofilm research. Nat Rev Microbiol 5(1):76–81
Sonkusale KD, Tale VS (2015) Isolation and characterization of biofilm forming bacteria from oral microflora. Int J Curr Microbiol App Sci 2:118–127
Sravanakumar G, Durgaprasad YV, Ramana T, Devi CS (2014) Isolation and identification of bacteria from marine biofilm on the artificial plat forms (iron panels) from Visakhapatnam Coast, India. Indian Journal of Geo-Marine Sciences 43(6):955–959
Kavitha S, Raghavan V (2018) Isolation and characterization of marine biofilm forming bacteria from a ship’s hull. Fron Biol 13(3):208–214
Kumaran S, Radhakrishnan M, Balagurunathan R (2011) Potential bioactive compound from marine actinomycetes against biofouling bacteria. J Adv Biotech 10:22–26
Gopikrishnan V, Pazhanimurugan R, Shanmugasundaram T, Radhakrishnan M, Balagurunathan R (2013) Bioprospecting of actinobacteria from mangrove and estuarine sediments for antifouling compounds. Int J Innov Res Sci Eng Technol 2(7):2726–2735
Waturangi DE, Hariyanto JP, Lois W, Hutagalung RA, Hwang JK (2017) Inhibition of marine biofouling by aquatic actinobacteria and coral-associated marine bacteria. Malaysian Journal of Microbiology 92–99. https://doi.org/10.21161/mjm.86016
Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, Zodape ST, Patolia JS (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South Afr J Bot 75(2):351–355
Bokil KK, Mehta VC, Datar DS (1972) Seaweed as manure III. Field manurial trials on Pennisetum typhoides S. H. (Pearl Millet) and Arachis hypogea (Groundnut). Bot Mar 15(3):148–150
Layek J, Das A, Idapuganti RG, Sarkar D, Ghosh A, Zodape ST, Lal R, Yadav GS, Panwar AS, Ngachan S, Meena RS (2018) Seaweed extract as organic bio-stimulant improves productivity and quality of rice in eastern Himalayas. J Appl Phycol 30(1):547–558
Thomas KV, Brooks S (2010) The environmental fate and effects of antifouling paint biocides. Biofoul 26(1):73–88
Satheesh S, Ba-akdah MA, Al-Sofyani AA (2016) Natural antifouling compound production by microbes associated with marine macroorganisms—a review. Electr J Biotech 21:26–35
Ogasawara K, Yamada K, Hatsugai N, Imada C, Nishimura M (2016) Hexose oxidase-mediated hydrogen peroxide as a mechanism for the antibacterial activity in the red seaweed Ptilophora subcostata. PloS one 11(2):0149084
Patra JK, Das G, Baek KH (2015) Chemical composition and antioxidant and antibacterial activities of an essential oil extracted from an edible seaweed. Laminaria japonica L Molecul 20(7):12093–12113
Iyapparaj P, Ramasubburayan R, Raman T, Das N, Kumar P, Palavesam A, Immanuel G (2012) Evidence for the antifouling potentials of marine macroalgae Sargassum wightii. Adv Nat Appl Sci 6(2):153–163
Ara J (2001) Studies on the bioactivity and elementology of marine algae from the coast of Karachi, Pakistan. Phyto Res 18(11):865–872. https://doi.org/10.1002/ptr.1441
Rizvi MA, Shameel MU (2004) Biological activity and elementology of benthic algae from Karachi coast. Pak J Bot 35(5):717–730
Sastry VM, Rao GR (1995) Dioctyl phthalate, and antibacterial compound from the marine brown alga—Sargassum wightii. J Appl Phycol 7(2):185–186
Chambers LD, Stokes KR, Walsh FC, Wood RJ (2006) Modern approaches to marine antifouling coatings. Surf Coat Tech 201(6):3642–3652
Wilsanand V, Wagh AB, Bapuji M (1999) Antibacterial activities of anthozoan corals on some marine microfoulers. Microbios 99(394):137–145
Rittschof D, Clare AS, Gerhart DJ, Mary SA, Bonaventura J (1992) Barnacle in vitro assays for biologically active substances: toxicity and settlement inhibition assays using mass cultured Balanus amphitrite Darwin. Biofoul 6(2):115–122
Hellio C, Bremer G, Pons AM, Le Gal Y, Bourgougnon N (2000) Inhibition of the development of microorganisms (bacteria and fungi) by extracts of marine algae from Brittany. France Appl Microbio Biotech 54(4):543–549
Tranchida PQ, Mondello L (2012) Current-day employment of the micro-bore open-tubular capillary column in the gas chromatography field. J Chromato A 1261:23–36
Gadhi AA, El-Sherbiny MM, Al-Sofyani AM, Ba-Akdah MA, Satheesh S (2018) Antibiofilm activities of extracts of the macroalga Halimeda sp. from the red sea. J Mar Sci Tech 26(6):8
Kang JY, Bangoura I, Cho JY, Joo J, Choi YS, Hwang DS, Hong YK (2016) Antifouling effects of the periostracum on algal spore settlement in the mussel Mytilus edulis. Fish Aqua Sci 19(1):1–6
Deepak P, Sowmiya R, Kamaraj C, Josebin MP, Aiswarya D, Balasubramani G, Amutha V, Perumal P (2018) Gc-Ms profiling, chemical characterization, antioxidant, Î ‘-amylase And Î ‘-glucosidase inhibition of selected seaweeds from southeast coast of India: an in vitro approach. J Drug Del Therap 8(2):60–72
Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, Lipton AP (2009) Antimicrobial potential and seasonality of red algae collected from the southwest coast of India tested against shrimp, human and phytopathogens. Annals Microbio 59(2):207–219
Kelecom A (2002) Secondary metabolites from marine microorganisms. An Acad Bras Ciênc 74(1):151–170
Baleta FN, Bolaños JM, Ruma OC, Baleta AN, Cairel JD (2017) Phytochemicals screening and antimicrobial properties of Sargassum oligocystum and Sargassum crassifolium Extracts. J Med Plants 5(1):382–387
Manilal A, Sujith S, Sabarathnam B, Kiran GS, Selvin J, Shakir C, Lipton AP (2010) Antifouling potentials of seaweeds collected from the southwest coast of India. World J Agri Sci 6(3):243–248
Acknowledgements
The corresponding author MK is thankful to the Saveetha School of Engineering, SIMATS Deemed University, Chennai, for providing the lab facilities for doing research work, and ME is thankful to CAS in Marine Biology, Annamalai University, for providing the lab facilities for doing research work.
Author information
Authors and Affiliations
Contributions
M. Kavisri: conceptualization, supervision, data curation, review & editing, funding acquisition, writing—original draft. M. Elangovan: methodology, investigation, resources and formal analysis: conceptualization, supervision, review & editing P. Anantharaman and Ragi: supervision, review & editing.
Corresponding author
Ethics declarations
Ethical approval
In this study, the animal experiment was not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fouling microbes were isolated from panels and identified by molecular level.
• Secondary metabolites have shown non-toxic anti micro and macro fouling effect in lower dose.
• Several active metabolites were quantified and identified by phytochemical screening and GC-MC analysis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manickam Elangovan, Perumal Anantharaman & M. Kavisri Eco-friendly antifoulants from seaweeds by in vitro and in vivo experiments and secondary metabolites profiling. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04485-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04485-2