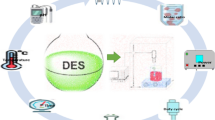
Abstract
Volatile fatty acids (VFAs) such as acetic acid have a wide range of applications with high demand. Since acetic acid is well soluble in water, treating its wastewater presents a technical challenge. In this work, new hydrophobic deep eutectic solvents (DESs) were synthesized by using methyltrioctylammonium chloride (TOMAC) as hydrogen bond acceptor (HBA) and acetic acid as hydrogen bond donor (HBD). The properties of DESs were characterized by DSC, TG, and FT-IR. As HBA, TOMAC was used for in situ generation of DES to extract acetic acid from aqueous solution. The extraction efficiency reached 65.28% when TOMAC/acetic acid was 7:3 (molar ratio), which is better than that of the traditional acetic acid extractants, such as tributyl phosphate (60.46%). When metal ions such as Ca2+, Mg2+, and Fe3+ coexist in solution, the salt precipitation effect not only prevents the emulsification phenomenon but also improves the acetic acid extraction effect. For volatile fatty acids such as propionic acid, butyric acid, and valeric acid, the extraction efficiency of TOMAC was good, reaching 84.96%, 92.04%, and 95.81%, respectively. The method of using TOMAC to extract acetic acid from aqueous solutions by forming DES in situ provides a new way for the treatment of VFAs wastewater.
Graphical Abstract






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sarchami T, Batta N, Berruti F (2021) Production and separation of acetic acid from pyrolysis oil of lignocellulosic biomass: a review. Biofuel Bioprod Biorefin 15:1912–1937. https://doi.org/10.1002/bbb.2273
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2023) Novel solvent properties of choline chloride/urea mixtures. Chem Comm 1:70–71. https://doi.org/10.1039/B210714G
Abbasi NM, Farooq MQ, Anderson JL (2021) Modulating solvation interactions of deep eutectic solvents formed by ammonium salts and carboxylic acids through varying the molar ratio of hydrogen bond donor and acceptor. J Chromatogr A 1643:462011. https://doi.org/10.1016/j.chroma.2021.462011
Noé FM, Vicente DA (2021) Organocatalytic transformations in deep eutectic solvents: green methodologies made greener. Tetrahedron 84:131967. https://doi.org/10.1016/j.tet.2021.131967
van Osch DJGP, Zubeir LF, Bruinhorst AVD, Rocha MAA, Kroon MC (2015) Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem 17:4518–4521. https://doi.org/10.1039/C5GC01451D
Hu XZ, Zhang LY, Xia H, Peng M, Zhou YX, Xu ZM, Peng XT (2021) Dispersive liquid-liquid microextraction based on a new hydrophobic deep eutectic solvent for the determination of phenolic compounds in environmental water samples. J Sep Sci 44:1510–1520. https://doi.org/10.1002/jssc.202001055
Florindo C, Nathalie VM, Bernardo DR, Branco LC, Marrucho IM (2019) Hydrophobic deep eutectic solvents for purification of water contaminated with Bisphenol-A. J Mol Liq. 297:111841. https://doi.org/10.1016/j.molliq.2019.111841
Darwish AS, Warrag SEE, Lemaoui T, Alseiari MK, Hatab FA, Rafay R, Alnashef I, Rodríguez J, Alamoodi N (2021) Green extraction of volatile fatty acids from fermented wastewater using hydrophobic deep eutectic solvents. Fermentation 7:226. https://doi.org/10.3390/fermentation7040226
Aşçı YS, Lalikoglu M (2021) Development of new hydrophobic deep eutectic solvents based on trioctylphosphine oxide for reactive extraction of carboxylic acids. Ind Eng Chem Res 60:1356–1365. https://doi.org/10.1021/acs.iecr.0c04551
Zinov’eva IV, Kozhevnikova AV, Milevskii NA, Zakhodyaeva YA, Voshkin AA (2022) Extraction of Cu(II), Ni(II), and Al(III) with the deep eutectic solvent D2EHPA/Menthol. Theor Found Chem Eng 56:221–229. https://doi.org/10.1134/S0040579522020178
Liu RH, Geng YQ, Tian ZJ, Wang N, Wang M, Zhang GJ, Yang YZ (2021) Extraction of platinum(IV) by hydrophobic deep eutectic solvents based on trioctylphosphine oxide. Hydrometallurgy 199:105521. https://doi.org/10.1016/j.hydromet.2020.105521
Cheng HY, Qi ZW (2020) Research progress of deep eutectic solvents for extraction and separation. Chem Prog 39:4896–4907. https://doi.org/10.16085/j.issn.1000-6613.2020-0956
Qin L, Li JS, Cheng HY, Chen LF, Qi ZW, Yuan WK (2017) Association extraction for vitamin E recovery from deodorizer distillate by in situ formation of deep eutectic solvent. AIChE J 63:2212–2220. https://doi.org/10.1002/aic.15606
Pang K, Hou Y, Wu WZ, Guo WJ, Kenneth NM (2012) Efficient separation of phenols from oils via forming deep eutectic solvents. Green Chem 14:2398–2401. https://doi.org/10.1039/C2GC35400D
Zhang Y, Li ZY, Wang HY, Xuan XP, Wang JJ (2016) Efficient separation of phenolic compounds from model oil by the formation of choline derivative-based deep eutectic solvents. Sep Purif Technol 163:310–318. https://doi.org/10.1016/j.seppur.2016.03.014
Shishov A, Nechaeva D, Bulatov A (2019) HPLC-MS/MS determination of non-steroidal anti-inflammatory drugs in bovine milk based on simultaneous deep eutectic solvents formation and its solidification. Microchem J 150:104080. https://doi.org/10.1016/j.microc.2019.104080
Shi YY, Li X, Shang Y, Li TM, Zhang KG, Fan J (2020) Effective extraction of fluorescent brightener 52 from foods by in situ formation of hydrophobic deep eutectic solvent. Food Chem 311:125870. https://doi.org/10.1016/j.foodchem.2019.125870
Li JWJ, Wang JW, Wu MY, Cheng HY, Chen LF, Qi ZW (2020) Deep deterpenation of citrus essential oils intensified by in situ formation of a deep eutectic solvent in associative extraction. Ind Eng Chem Res 59:9223–9232. https://doi.org/10.1021/acs.iecr.0c00442
Liu LJ, Su BL, Wei QF, Ren XL (2022) Separation of lactic acid based on amide-based hydrophobic deep eutectic solvents: Insights from experiments and molecular dynamics simulations. ACS Sustain Chem Eng 10:15589–15598. https://doi.org/10.1021/acssuschemeng.2c05079
Stefanovic R, Ludwig M, Webber GB, Atkin R, Page AJ (2017) Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys Chem Chem Phys 19:3297–3306. https://doi.org/10.1039/C6CP07932F
Milevskii NA, Zinov’eva IV, Zakhodyaeva YuA, Voshkin AA (2022) Separation of Li(I), Co(II), Ni(II), Mn(II), and Fe(III) from hydrochloric acid solution using a menthol-based hydrophobic deep eutectic solvent. Hydrometallurgy 207:105777. https://doi.org/10.1016/j.hydromet.2021.105777
Flakus HT, Hachuła B (2011) The source of similarity of the IR spectra of acetic acid in the liquid and solid-state phases. Vib Spectrosc 56:170–176. https://doi.org/10.1016/j.vibspec.2011.02.001
Flakus HT, Tyl A (2007) Polarized IR spectra of the hydrogen bond in acetic acid crystals. Chem Phys 336:36–50. https://doi.org/10.1016/j.chemphys.2007.05.005
Wang YM, He Y, Yin SH, Long HL, Li SW (2020) Research on extraction of zinc from spent pickling solution using Aliquat 336. Hydrometallurgy 193:105322. https://doi.org/10.1016/j.hydromet.2020.105322
Luo DS, Huang J, Zhang YM, Hong Liu H, Pengcheng Hu (2020) Highly efficient separation and extraction of vanadium from a multi-impurity leachate of vanadium shale using tri-n-octylmethylammonium chloride. Sep Purif Technol 230:115842. https://doi.org/10.1016/j.seppur.2019.115842
Bai YE, Zhang XT, Zhang RM, Hou J, Niu YJ, Hu S, Gao JP (2021) Simultaneous determination of lobetyolin and atractylenolide III in Codonopsis Radix by dispersive liquid-liquid microextraction based on hydrophobic deep eutectic solvent. Microchem J 170:106664. https://doi.org/10.1016/j.microc.2021.106664
Chen WJ, Xue ZM, Wang JF, Jiang JY, Zhao XF, Mu TC (2018) Thermal stability study of deep eutectic solvents. Acta Phys Chim Sin 34:904–911. https://doi.org/10.3866/PKU.WHXB201712281
Janković B, Manić N, Perović I, Vujković M, Zdolšek N (2023) Thermal decomposition kinetics of deep eutectic solvent (DES) based on choline chloride and magnesium chloride hexahydrate: New details on the reaction mechanism and enthalpy–entropy compensation (EEC). J Mol Liq 374:121274. https://doi.org/10.1016/j.molliq.2023.121274
Frolkova AV, Frolkova AK, Gaganov IS (2022) Comparison of extractive and heteroazeotropic distillation of high-boiling aqueous mixtures. ChemEngineering 6:83. https://doi.org/10.3390/chemengineering6050083
Makoś P, Słupek E, Gębicki J (2020) Hydrophobic deep eutectic solvents in microextraction techniques–a review. Microchem J 152:104384. https://doi.org/10.1016/j.microc.2019.104384
Diego RL, Arkaitz B, Gonzalo PM, Pablo NV, Ismael ÁV, José AD, Silvia AT, Juan G, Marcos L (2019) Sustainable recovery of volatile fatty acids from aqueous solutions using terpenoids and eutectic solvents. ACS Sustain. Chem Eng 7:16786–16794. https://doi.org/10.1021/acssuschemeng.9b04290
Jia KJ, Zhang T, Feng LB, Zhang C, Xu X, Xiong JM (2021) Liquid-liquid equilibrium study for ternary system of (water+acetic acid+2-heptanol). J Chem Thermodyn 153:106305. https://doi.org/10.1016/j.jct.2020.106305
Shakya AK, Jaiswal Y, Pal SL, Srivastava CH (2022) Reactive extraction of acetic acid by using tri-butyl-phosphate with different diluents. Chem Data Collect 39:100855. https://doi.org/10.1016/j.cdc.2022.100855
Alves-Lima DF, Rodrigues CF, Pinheiro CT, Gando-Ferreira LM, Quina MJ, Ferreira AG (2022) Highly selective solvent extraction of Zn(II) and Cr(III) with trioctylmethylammonium chloride ionic liquid. Can J Chem Eng 100:131–142. https://doi.org/10.1002/cjce.24051
Cheng JH, Lu T, Wu X, Zhang HJ, Zhang CY, Peng CA, Shouqiang Huang SQ (2019) Extraction of cobalt(II) by methyltrioctylammonium chloride in nickel(II)-containing chloride solution from spent lithium ion batteries[J]. RSC Adv 9:22729–22739. https://doi.org/10.1039/C9RA02719J
Lommelen R, Onghena B, Binnemans K (2020) Cation effect of chloride salting agents on transition metal ion hydration and solvent extraction by the basic extractant methyltrioctylammonium chloride. Inorg Chem 59:13442–13452. https://doi.org/10.1021/acs.inorgchem.0c01821
Zhang XK, Zhou KG, Lei QY, Y, Peng CH, Chen W (2019) Selective removal of Iron from acid leachate of red mud by Aliquat 336. JOM 71:4608–4615 . 10. 1007/s11837–019–03801–4.
Mishra RK, Rout PC, Sarangi K, Nathsarma KC (2011) Solvent extraction of Fe(III) from the chloride leach liquor of low grade iron ore tailings using Aliquat 336. Hydrometallurgy 108:93–99. https://doi.org/10.1016/j.hydromet.2011.03.003
Wei W, Cho CW, Kim S, Song MY, Bediako JK, Yun YS (2016) Selective recovery of Au(III), Pt(IV), and Pd(II) from aqueous solutions by liquid–liquid extraction using ionic liquid Aliquat-336. J Mol Liq 216:18–24. https://doi.org/10.1016/j.molliq.2016.01.016
Fernandes CC, Haghbakhsh R, Marques R, Paiva A, Carlyle L, Duarte ARC (2021) Evaluation of deep eutectic systems as an alternative to solvents in painting conservation. ACS Sustain Chem Eng 9:15451–15460. https://doi.org/10.1021/acssuschemeng.1c04591
Van Krevelen DW (2009) Te Nijenhuis K. Elsevier, Properties of Polymers
Funding
This study was funded by the National Natural Science Foundation of China (22178113).
Author information
Authors and Affiliations
Contributions
Linchao Zhu (First Author): Investigation, Writing-original draft.
Lin Wang: Conceptualization, Resources.
Peiqing Yuan: Data curation.
Xinru Xu: Validation.
Jingyi Yang (Corresponding Author): Project administration, Supervision, Writing-review editing.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, L., Wang, L., Yuan, P. et al. Extraction of volatile fatty acids from aqueous solution by in situ formed deep eutectic solvent with methyltrioctylammonium chloride. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04289-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04289-4