Abstract
Activated carbon obtained from red algae Gracilaria changii was used as an adsorbent to remove Pb (II) and Cu (II) from an aqueous solution. The raw red algae were first impregnated with phosphoric acid, followed by thermal activation. The Box–Behnken design was used to optimize the activation process. The optimum activation parameters were 84%, 650 °C, and 175 min for acid concentration, activation temperature, and activation time, respectively. The obtained activated carbon had a high surface area of 867 m2/g. The removal of Pb (II) and Cu (II) was evaluated using a batch adsorption study. The effect of solution pH on the removal of metal ions was investigated within the range of 2–7. The effect of three important adsorption parameters (initial metal ion concentration, adsorbent dosage, and contact time) was analyzed using central composite design. The optimum removal of Pb (II) and Cu (II) was 76% and 36%, respectively. The adsorption kinetics obeyed the pseudo-second-order model, while the adsorption isotherm obeyed the Langmuir model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal pollution is a well-known global environmental issue due to its toxicity and persistence [1, 2]. Heavy metals are non-biodegradable elements that have many toxicological effects on human health and are detrimental to the environment. They can accumulate in living organisms, causing diverse health effects and eventually becoming fatal to humans [3,4,5,6].
The United States Environmental Protection Agency (USEPA) listed both Pb (II) and Cu (II) on the priority control pollutant list due to their persistence and irreversible toxicity [7]. Several techniques have been utilized to remove heavy metals from wastewater, including ion exchange, chemical precipitation, chemical coagulation, and membrane separation [8]. These techniques are often ineffective and very expensive when used to reduce heavy metal ions. Water containing heavy metals is produced in various industries, including petroleum, mining, and electroplating [9, 10]. Heavy metals can accumulate in the environment and enter the food chain directly or indirectly [5, 11, 12].
Different heavy metals bearing water treatment methods had been used, such as chemical precipitation, membrane filtration, coagulation, and ion exchange. However, the main limitations of these methods are ineffectiveness and/or high cost for low heavy metal concentrations. The complexity of operation and design is an additional drawback of such techniques [13].
On the other hand, adsorption is recognized as an economically effective heavy metal removal process due to its simplicity and flexibility in both operation and design [1, 10, 14]. A variety of adsorbents, like biochar [15, 16], MXenes [17, 18], graphene oxide [19], carbon nanotubes [20], zeolites [21, 22], spent mushroom substrate [23], and activated carbon [23, 24], were reported on the removal of heavy metals from aqueous solutions. Adsorption with activated carbon has proven to be very efficient and promising, especially when local biomass reduces costs and utilizes existing resources. Activated carbon adsorbents have a high surface area, various characteristics of surface chemistry, and a high degree of porosity resulting from their wide use in removing heavy metal contaminants.
Macroalgae or seaweeds are natural renewable biomass that can be found abundantly in coastal areas. Seaweeds can be considered as a low-cost material that exists abundantly in nature; is easy and fast to grow; needs no fertilizing, no weeding, and no watering; and has very few enemies in the form of pests or disease; hence, it requires little to no processing, and only a few resources are needed to be invested to grow a seaweed farm [25]. Activated carbon adsorbents have a high surface area, various characteristics of surface chemistry, and a high degree of porosity resulting from their wide use in removing heavy metal contaminants [26, 27]. High costs of activated carbon led to growing research aim to find cheaper alternatives. Carbonaceous materials that required little processing, by-products of another industry, or abundant in nature can be considered low-cost precursors [28]. These characteristics lead researchers recently to invest efforts into using it as a precursor to producing activated carbon. This study aimed to prepare activated carbon using the red algae Gracilaria changii as a precursor for removing Pb (II) and Cu (II) from water. Response surface methodology was used to investigate the preparation of activated carbon and batch adsorption studies. Various characterization techniques were utilized, including iodine number, BET, FTIR, and FESEM-EDX.
2 Material and methods
2.1 Preparation of activated carbon
G. changii was collected from the Fishery Department of Langkawi. The algae were washed thoroughly with tap water followed by distilled water for further purity. After washing, the algae were dried under direct sunlight for 2 days, followed by oven drying at 70 °C for 24 h. The dried red algae (DRA) were ground, sieved in the 0.15–0.30 mm range, and stored in an airtight bottle before activation.
The activation process involved two steps, impregnation with phosphoric acid and thermal activation (Fig. 1). In the first step, the dried biomass was mixed with phosphoric acid of different strengths (25–85%) with a mix ratio of 1 g/5 mL at room temperature until homogenous impregnation occurred. The mixture is then heated in an oven at a non-ramping heating rate of 5 °C/min to 105 °C for 24 h. The product was then placed in a tube furnace (OTF-1200X-80) (MTI Corporation, Richmond, California, CA, USA) and thermally activated under a nitrogen atmosphere at the desired temperature (400–800 °C) and residence time (60–180 min). The activated carbon was then washed with distilled water using a Soxhlet extractor. The product was then dried and stored in glass bottles.
The effects of acid concentration (A), activation temperature (B), and residence time (C) on the iodine number (R) were modeled and evaluated using RSM. The iodine number is a relative indicator of porosity in activated carbon. It can be used as an approximation of surface area and the relative activation level of activated carbon by adsorption of iodine from an aqueous solution [29]. The Box–Behnken design was implemented, and the factor limits are shown in Table 1. BBD was selected due to economic reasons as it is considered more labor efficient than central composite design (CCD) and much more than full factorial design (FFD) in designing the experiment. BBD is also useful to avoid experiments where all factors have extreme values. This is to avoid undesired phenomena that might occur in extreme conditions [30]. The number of experiments required to develop BBD was calculated using Eq. 1:
where K is the number of independent factors, N is the number of experiments runs, and C is the number of experiments carried out at the center. State–Ease design expert v10.0.1 was used to determine the experiment matrix. Analysis of variance (ANOVA) was used to analyze the model statistically.
2.2 Batch adsorption study
2.2.1 Synthetic solution preparation
Stock solutions of (1000 mg/L) of Pb (II) and Cu (II) were prepared by dissolving lead nitrate (1.598 g) or copper nitrate trihydrate (3.802 g) in 1 L of distilled water. The working solution was prepared by further dilution of the stock solutions.
2.2.2 Experimental procedure
The experiment proceeds essentially the same as that we used previously to study the biosorption of Pb (II) and Cu (II) onto dried algae biomass [31]. The batch adsorption study was conducted in 250-mL Erlenmeyer flasks that contain 100 mL of Pb (II) or Cu (II). Four parameters were investigated: pH, initial metal ion concentration, adsorbent dosage, and contact time. The samples were agitated at 180 rpm at room temperature for a predetermined period before they were collected and filtered through filter paper (Whatman No. 1, 0.45 µm). Metal ion concentrations were analyzed using atomic absorption spectroscopy. All tests were carried out in triplicate for optimum precision and minimal error. Equation 2 was used to calculate the removal efficiency [32]:
where Ci and Cf are the initial and final metal ion concentrations, respectively.
2.2.3 Experiment design
Solution pH effects
The effect of solution pH on the adsorption efficiency was investigated using a 100-mL solution of 100 mg/L of Pb (II) or Cu (II) and 1 g\L of activated carbon. The pH was varied in the range of 2–7 using hydrochloric acid and sodium hydroxide. The mixture was then agitated at room temperature for 120 min before being filtered and analyzed using AAS.
Experimental design using RSM
The effects of initial ion concentration (A), contact time (B), and adsorbent dosage (C) on the metal removal efficiency (R) were modeled and evaluated using central composite design. The number of experiments was calculated based on Eq. 3 as follows:
where N is the number of runs, K is the number of factors, and C is the number of experiments at the center. State–Ease design expert v10.0.1 was used to design the experiment. The ranges and levels of the independent factors are shown in Table 2. Analysis of variance (ANOVA) was used to evaluate the model statistically, and the interaction between the variables was studied using surface contour plots.
2.3 Characterization
2.3.1 Iodine number
The iodine number is a relative indicator of porosity in activated carbon. It can be used as an approximation of surface area and the relative activation level of activated carbon by adsorption of iodine from an aqueous solution [29]. The iodine number was determined according to ASTM D4607-14 [29]. The test is based on a three-point adsorption isotherm. Three different weights of adsorbent are mixed with 25 mL of standard iodine solution 0.100 N under specific conditions for 30 s. The solution is then filtered, and the residual iodine is measured by titration with standard sodium thiosulfate 0.100 N. The amount of iodine adsorbed (mg/g) at a residual iodine concentration of 0.02 N is reported as the iodine number. The amount of iodine number was determined using Eq. 4 [33]:
where V is iodine solution volume, 25 mL, Ti is sodium thiosulfate used for titration of 10 mL iodine solution, Tf is sodium thiosulfate used for titration of 10 mL iodine filtrate, g is the weight of activated carbon, Mi is the molar weight of iodine = 126.9044, and C is concentration of iodine solution 0.100 N.
2.3.2 Determination of total surface area
The Brunauer–Emmett–Teller (BET) surface area and average pore diameter were measured using Micromeritics ASAP 2020 v3.04 H. The nitrogen gas was used as an adsorbate during the testing. The gas thermal conductivity was used to monitor the adsorption/desorption process. Micropore volume and total surface area were determined by fitting the data to the Brunauer–Emmett–Teller (BET) equation. The nitrogen gas passing at relative pressures was used to evaluate the pore volume of the sample.
2.3.3 Determination of elemental composition
Both elements and their oxides of the dried seaweeds and the activated carbon were quantitatively analyzed using X-ray fluorescent (XRF) spectrometry (Model S8, Tiger).
2.3.4 FESEM-EDX analysis
Field emission scanning electron microscopy with energy-dispersive X-ray spectroscopy was used to study and analyze the surface morphology of the adsorbent. The materials were subjected to analysis before and after activation as well as after the adsorption of Pb (II) and Cu (II).
2.3.5 FTIR analysis
The variation in the vibration frequency of functional groups before and after activation as well as before and after the adsorption process was measured using Fourier transform infrared (FTIR) spectroscopy. Infrared spectra were recorded within the range of 550–4000 cm−1. Essential FTIR v3.5 was used to process and analyze the spectral data.
3 Results and discussion
3.1 Adsorbent preparation
3.1.1 Model development for iodine number
A BBD was utilized to study and analyze the effects of acid concentration, activation temperature, and residence time on the activated carbon iodine number. The experiment design and the obtained results are shown in Table 3. The model equation was selected based on the highest polynomial order where the model was not aliased, and the additional terms were significant. A quadratic model was selected as suggested by the software due to the high order of polynomials. The final empirical equation of the model in terms of the coded factors is represented as follows:
where the coefficients with A, B, and C represented the effect of acid concentration, activation temperature, and activation time, respectively. The coefficients with two factors (AB, AC, BC) and (B2, C2) represented the interaction between the two parameters and the quadratic effect. The negative sign in front of the term indicates an antagonistic effect, while the positive sign indicates a synergistic effect [34].
The analysis of variance (ANOVA) was utilized to measure the model significance and determine the relationship between the independent variable and the response. The results of the statistical analysis are shown in Table 4. The P and F values evaluate the factor coefficient’s regression and significance, respectively. The significance of the model is confirmed when the model F value of 181 and Prob > F is less than 0.0001. The term is considered significant if the value of the P value < 0.05. From the ANOVA table, it can be concluded that the independent factors (acid concentration, activation temperature, and activation time) are significant terms for the iodine number. The interaction terms of AB, BC, and AC and the quadratic terms of B2 and C2 were also significant for the iodine number model. No significant lack of fit indicates that the model fits the obtained data well [35].
The model was also assessed with other descriptive statistics shown in Table 5. The correlation coefficient (R2) was used to evaluate the quality of the model approximation to the actual experimental data. The R2 value close to unity is desirable and was in reasonable agreement with the adjusted R2 [36]. The term adequate precision (AP) ratio refers to the response variable concerning the target under varying noise conditions. An AP value higher than 4 is desirable [37]. The reproducibility of the model is indicated by the coefficient of variance (CV). It is measured as the ratio of the stand error of the estimate to the mean value of the response. The model is considered reproducible if the value is less than 10% [31, 38].
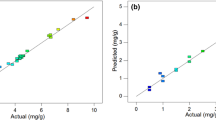
The residual vs. predicted plot was utilized to diagnose the adequacy of the developed model. The scatter plot of studentized residuals vs. predicted points is shown in Fig. 2A. The data points should be randomly scattered, indicating constant variance of experimental observations and adequacy of the quadratic model. The data points were in the range of ± 3, implying that no response transformation is needed for the experimental design [39]. A plot of predicted versus experimental data was generated to observe the model performance and adequacy of the predicted iodine number within the experimental range. The predicted points are close to their experimental values implying good model performance, as shown in Fig. 2B.
3.1.2 Effects of preparation factors on the iodine number
Based on the P values shown in Table 4, it can be observed that both acid concentration, activation temperature, and time significantly affect the adsorption of iodine number. It was also concluded that both A and B have the most significant effect on the iodine number, while time exhibits the lowest effect.
Figure 3 represents the three-dimensional response surface and contour plots which show the effect of combined effects of significant factors on the iodine number of the produced activation carbon. The graph shows that it has been a gradual increase in the iodine number as the acid concentration increases (Fig. 3a). The existence of phosphoric acid in the lignocellulosic precursors’ interiors prevents particle shrinkage by inhibiting the formation of tar and other liquids and forming cross-links, thereby protecting the formation of the pores, and reducing the pore-clogging, which can negatively affect the development of pore structure [40,41,42]. The graph also shows that the iodine number increased by increasing activation temperature (Fig. 3c). A higher iodine number relates to the pore development during the activation process. A slight decrease in the iodine number was obtained at a temperature higher than 650 °C, which might result from pore walls collapsing, thus resulting in a lower iodine number [43, 44]. On the other hand, activation time has the least effect on the iodine number within the design period with an F value of 6.03 (Fig. 3b and c) revealing that the iodine number increases gradually by the increment of time. This increase might be due to the increment of reaction between the phosphoric acid and the biomass, thus enhancing the development of pore structure and the formation of many active sites [37].
Three-dimensional response and contour plots for combined effects on RAA iodine number. a Effect of acid concentration and activation temperature, activation time (120 min). b Effect of acid concentration and activation time, temperature (600 °C). c Effect of activation temperature and activation time, acid concentration (55%)
In order to optimize the iodine number of the produced activated carbon, the numerical optimization menu was selected using Design Expert software v10.0.1 (Stat-Ease Inc., Minneapolis). Optimization criteria were set as maximum values for iodine number. The values of the three variables (acid concentration, activation temperature, and activation time) were set to be in the design ranges. The optimum experimental conditions were selected with the highest desirability value. Table 6 shows the predicted and experimental values for the software proposed preparation condition. Since the predicted and experimental values deviated less than 10%, the model was concluded to be suitable enough to determine the iodine number.
3.2 Characterization
3.2.1 Elemental compositions of DRA and RAA
The elemental compositions of DRA and RAA were analyzed using X-ray fluorescence spectrometry. The elements and their oxide percentages are shown in Table 7. It can be observed that calcium and manganese constitute the largest percentage of the raw algae. It can be observed that after activation, there is less amount of SO3, CaO, and MnO, and a larger portion of the material consisted of P2O5. The reduction of these elements’ oxides might be due to the high activation temperature and release of gasses and liquids. The formation of P2O5 is due to the reaction of phosphoric acid with the biomass during impregnation and thermal activation at high temperatures.
3.2.2 Surface area and pore characterization for DRA and RAA
BET surface area and average pore diameter were measured using Micromeritics ASAP 2020 v3.04 H. The results were analyzed by the software provided with the system. For DRA, the BET surface area was 3.36 m2/g which is relatively lower than some other biomass found in the literature. No micropores were found in the raw biomass. The average pore diameter was found to be 9.6 nm which falls into the mesopore category according to the International Union of Pure and Applied Chemistry (IUPAC). For RAA, the BET surface area was 867.82 m2/g. The significant increase in surface area is due to the chemical and thermal treatment. The results are relatively close to other algae-derived activated carbon found in the literature. The average pore diameter was 3.80 nm which also falls into the mesopore material category according to IUPAC. Table 8 shows the surface physical properties of both DRA and RAA and other activated carbon found in the literature.
3.2.3 FESEM analysis for DRA and RAA
The surface morphology of both raw Gracilaria changii and its activated carbon was examined using FESEM-EDX. Figure 4 and Fig. 5 show the surface morphology and composition obtained. It can be observed that the surface of raw biomass was rough with some minor pores. From Fig. 5, the pores’ surface morphology changes and pore development can be observed after activation. EDX analysis before and after the activation shows the attachment of P to the surface of activated carbon. EDX analysis also shows that some mineral was also released from the surface due to the activation process.
3.3 Batch adsorption study
3.3.1 Solution pH effect on adsorption
The solution pH affects the heavy metal adsorption process significantly. The adsorbent surface chemistry is heavily influenced by the solution pH [51, 52]. Low adsorption was observed at low pH due to the existence of hydrogen ions and their competition with metal ions for the adsorption sites [53]. Maximum removal percentages at pH 5 were 39% and 19% for Pb (II) and Cu (II), respectively. These results are similar to those reported in the literature for Pb (II) and Cu (II) removal by using different activated carbon [52, 54,55,56,57,58]. The effect of solution pH on the removal percentages is illustrated in Fig. 6.
3.3.2 Pb (II) and Cu (II) adsorption model development
CCD matrix was used to study the synergy effects of metal ion concentration (A), contact time (B), and adsorbent dosage (C), on the removal efficiency of Pb (II) and Cu (II). Table 9 shows the experimental design and the responses. The following equations were generated based on the results obtained:
ANOVA analysis for the equations and their corresponding regression coefficients are shown in Table 10. The statistical significance of each term in this table at 95% confidential index is indexed by its P value (< 0.05). If the magnitude of the P value is smaller than 0.05, the corresponding coefficient is highly significant [59]. The lack of fit test showed the variation of responses around the fitted model. Both models fit the data well because of the insignificant lack of fit. The coefficient values of variance (CV) were 5.75 and 7.62 for Pb (II) and Cu (II) models, respectively, which indicate that the models are reproducible [37]. The ratio of signal to noise (adequate precision) value was higher than four means that the models are valid to navigate the design space [60].
3.3.3 The synergistic effects of factors on Pb (II) and Cu (II) adsorption
Figure 7 and Fig. 8 show the three-dimensional surface plot for the effects and interactions of the independent variable on the removal efficiency of Pb (II) and Cu (II), respectively. The increase in initial metal ion concentration led to a decrease in the removal efficiency. This decrease can be explained by the limited activation sites available on the adsorbent [61]. Increasing the activated carbon dosage showed an increase in the removal efficiency, which can be explained justified by the presence of additional active sites and the large surface area available for adsorption [62]. The slight increase in the removal efficiency due to contact time indicated that the adsorption had reached equilibrium earlier, and the adsorption rate was rapid [52, 59].
Table 11 shows the predicted and experimental maximum Pb (II) and Cu (II) removal percentages achieved in optimum conditions. The models predicted that the maximum removal efficiencies are 76% and 35% for Pb (II) and Cu (II), respectively. Verification experiments were conducted and are in good agreement with the model results indicating the suitability and accuracy of the models. Figure 9 shows predicted versus actual values for Pb (II) and Cu (II) adsorption. Based on the plots, all the response values estimated by the models are in good agreement with the real values in the ranges of the independent variables. The metal uptakes obtained under the optimum conditions were 30.4 and 14.46 mg/g for Pb (II) and Cu (II), respectively. Table 12 lists the comparison of metal uptake of Pb (II) and Cu (II) onto various types of adsorbents.
3.3.4 FESEM-EDX analysis
FESEM-EDX was used to investigate the surface morphology and elemental composition of the activated carbon before and after the adsorption of Pb (II) and Cu (II) (Fig. 10). A significant difference can be observed in the morphological characterization of the adsorbent. The existence of smooth pores after the adsorption can be attributed to metal ion accumulation on the surface due to the electrostatic attraction effect [10, 70]. For the EDX analysis, the system was calibrated using CaCO3 (C), SiO2 (O and Si), MAD-10 feldspar (K), O (GaP), wollastonite (Ca), KCl (Cl), PbF2 (Pb), and Cu as standards. The presence of Pb and Cu peaks on the spectrums confirms the attachment of the metal ions on the surface. The decrease or disappearance of K, Ca, Si, and Cl from the spectrums indicates the possibility of ion exchange occurrence during the adsorption process [71,72,73].
3.3.5 FTIR analysis
FTIR spectrum analysis was used to study the functional groups available on the adsorbent surface and their interaction after the adsorption of Pb (II) and Cu (II). The spectrum peaks before and after the adsorption of heavy metals are shown in Fig. 11. Hydroxy stretching and carboxylic groups on the surface are indicated by the overlapping of O–H and N–H stretching at 3408.96 cm−1 [74, 75]. The peak at 1611.98 was assigned to C = C stretching [76], while C–H bonding was attributed to the peak found at 1413.01 cm−1 [77]. The peak at 1182.86 cm−1 corresponds to the C–O stretching [70]. The peak observed at 1054.07 cm−1 was assigned to the sulfoxide band [78, 79]. Table 13 summarizes the change in peaks before and after the adsorption. The shifts in these peaks after the adsorption of Pb (II) and Cu (II) show the involvement of these functional groups in the process [10, 80].
3.3.6 Adsorption isotherm study
The adsorption isotherms can be used to describe the relationship between the ion concentration and the number of ions adsorbed per unit mass of the adsorbent in the equilibrium solution at constant temperate [81]. The experimental data were assessed using Langmuir and Freundlich isotherm models. Table 14 shows the Langmuir and Freundlich isotherm constants for Pb (II) and Cu (II). As shown in Fig. 12, the experimental data fitted best in the Langmuir isotherm model for Pb (II) and Cu (II) with R2 greater than 0.99. The Langmuir isotherm assumes the creation of a homogenous monolayer of metal ions on the outer surface of the adsorbent [80]. The maximum sorption capacity was higher for Pb (II) than that for Cu (II). The KL value indicates the ions’ affinity to the binding sites available on the adsorbent surface. The values of RL in the range of 0–1 confirm that the sorption of Pb (II) and Cu (II) onto the red algae-activated carbon is favorable [82].
3.3.7 Adsorption kinetic study
The kinetic study for Pb (II) and Cu (II) adsorption onto red algae-activated carbon was conducted with both pseudo-first-order and pseudo-second-order kinetic models. Table 15 shows the kinetic model parameter correlation coefficients (R2), while kinetic model plots are shown in Fig. 13. The kinetic results obeyed the pseudo-second-order model with a correlation coefficient close to unity indicating the chemosorption type of sorption for Pb (II) and Cu (II).
4 Conclusion
Gracilaria changii was used as a raw material to be converted into activated carbon by chemical and thermal activation. The experimental design for activated carbon preparation was based on the Box–Behnken design revealing that phosphoric acid concentration, activation temperature, and activation time were important factors influencing the activated carbon iodine number. All parameters were found significant, with acid concentration having the highest impact on the iodine number. The optimal acid concentration, temperature, and time were 84%, 650 °C, and 175 min, respectively. The characterization results showed that the surface area of raw algae and activated carbons were 3.36 and 867.82 m2/g, respectively. The average pore diameter on the surface of activated carbon was 9.6 nm which falls into the mesopore category according to the International Union of Pure and Applied Chemistry (IUPAC).
Batch experiments were carried out for Pb (II) and Cu (II) ion adsorption onto the red algae-activated carbon. The study shows that the adsorption is pH-dependent, and the optimum pH is 5 for both Pb (II) and Cu (II). RSM was used to investigate initial ion concentration, contact time, and adsorbent dosage effects on the removal process. Maximum removal efficiencies were 76% and 36% at an initial concentration of 40 mg/L, contact time of 45 and 115 min, and adsorbent dosage of 1 g/L for Pb (II) and Cu (II), respectively. The kinetics of adsorption obeyed the pseudo-second order, while the adsorption isotherm obeyed the Langmuir model. The overall outcome of this investigation suggests the potential application of red algae Gracilaria changii activated carbon to remove heavy metals from water and wastewater.
This work shows that red algae Gracilaria changii may be successfully valorized through chemical activation using phosphoric acid to obtain activated carbon for the removal of Pb (II) and Cu (II) ions from aqueous solutions. G. changii proved to be a good low-cost sustainable source to be used as a precursor for activated carbon preparation. The prepared activated carbon has a relatively high surface area in comparison to other activated carbon products from various biomass sources.
Data availability
Data is available on request from the authors.
References
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Ho SH, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589. https://doi.org/10.1016/j.jclepro.2021.126589
Chen WH, Hoang AT, Nižetić S, Pandey A, Cheng CK, Luque R, Ong HC, Thomas S, Nguyen XP (2022) Biomass-derived biochar: from production to application in removing heavy metal-contaminated water. Process Saf Environ Prot 160:704–733. https://doi.org/10.1016/J.PSEP.2022.02.061
Sankaran R, Show PL, Ooi C-W, Ling TC, Shu-Jen C, Chen S-Y, Chang Y-K (2020) Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol Environ Policy 22(4):773–786
Kiran B, Kaushik A, Kaushik C (2007) Biosorption of Cr (VI) by native isolate of Lyngbya putealis (HH-15) in the presence of salts. J Hazard Mater 141(3):662–667. https://doi.org/10.1016/j.jhazmat.2006.07.026
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Malikula RS, Kaonga CC, Mapoma HWT, Chiipa P, Thulu FGD (2022) Heavy metals and nutrients loads in water, soil, and crops irrigated with effluent from WWTPs in Blantyre City. Malawi Water 14(1):121. https://doi.org/10.3390/W14010121
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231. https://doi.org/10.1016/j.jtemb.2019.05.003
Chakraborty R, Asthana A, Singh AK, Jain B, Susan ABH (2022) Adsorption of heavy metal ions by various low-cost adsorbents: a review. Environ Anal Chem 102(2):342–379. https://doi.org/10.1080/03067319.2020.1722811
Blázquez G, Martín-Lara MA, Tenorio G, Calero M (2011) Batch biosorption of lead (II) from aqueous solutions by olive tree pruning waste: equilibrium, kinetics and thermodynamic study. Chem Eng J 168(1):170–177. https://doi.org/10.1016/j.cej.2010.12.059
Verma A, Kumar S, Kumar S (2016) Biosorption of lead ions from the aqueous solution by Sargassum filipendula: equilibrium and kinetic studies. J Environ Chem Eng 4(4):4587–4599. https://doi.org/10.1016/j.jece.2016.10.026
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216. https://doi.org/10.1007/s10311-010-0297-8
Tchounwou P B, Yedjou C G, Patlolla A K, Sutton D J (2012) Heavy metal toxicity and the environment. In Molecular, clinical and environmental toxicology: volume 3: environmental toxicology; Luch, A., Ed.; Springer Basel: Basel; pp 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Shi T, Xie Z, Mo X, Feng Y, Peng T, Song D (2022) Highly efficient adsorption of heavy metals and cationic dyes by smart functionalized sodium alginate hydrogels. 8(6):343. https://doi.org/10.3390/GELS8060343
Park CM, Han J, Chu KH, Al-Hamadani YAJ, Her N, Heo J, Yoon Y (2017) Influence of solution PH, ionic strength, and humic acid on cadmium adsorption onto activated biochar: experiment and modeling. J Ind Eng Chem 48:186–193. https://doi.org/10.1016/j.jiec.2016.12.038
Wang Q, Wang B, Ma Y, Zhang X, Lyu W, Chen M (2022) Stabilization of heavy metals in biochar derived from plants in antimony mining area and its environmental implications. Environ Pollut 300:118902. https://doi.org/10.1016/J.ENVPOL.2022.118902
Jun B-M, Kim S, Heo J, Park CM, Her N, Jang M, Huang Y, Han J, Yoon Y (2019) Review of MXenes as new nanomaterials for energy storage/delivery and selected environmental applications. Nano Res 12(3):471–487. https://doi.org/10.1007/s12274-018-2225-3
Ahmaruzzaman MD (2022) MXene-based novel nanomaterials for remediation of aqueous environmental pollutants. Inorg Chem Commun 143:109705. https://doi.org/10.1016/J.INOCHE.2022.109705
Jun BM, Kim S, Kim Y, Her N, Heo J, Han J, Jang M, Park CM, Yoon Y (2019) Comprehensive evaluation on removal of lead by graphene oxide and metal organic framework. Chemosphere 231:82–92. https://doi.org/10.1016/j.chemosphere.2019.05.076
Fiyadh SS, AlSaadi MA, Jaafar WZ, AlOmar MK, Fayaed SS, Mohd NS, Hin LS, El-Shafie A (2019) Review on heavy metal adsorption processes by carbon nanotubes. J Clean Prod 230:783–793. https://doi.org/10.1016/j.jclepro.2019.05.154
Hong M, Yu L, Wang Y, Zhang J, Chen Z, Dong L, Zan Q (2018) Li R (2019) Heavy metal adsorption with zeolites: the role of hierarchical pore architecture. Chem Eng J 359:363–372. https://doi.org/10.1016/j.cej.2018.11.087
Li Y, Bai P, Yan Y, Yan W, Shi W, Xu R (2019) Removal of Zn 2+, Pb 2+, Cd 2+, and Cu 2+ from aqueous solution by synthetic clinoptilolite. Microporous Mesoporous Mater 273:203–211. https://doi.org/10.1016/j.micromeso.2018.07.010
Corral-Bobadilla M, González-Marcos A, Vergara-González EP, Alba-Elías F (2019) Bioremediation of wastewater to remove heavy metals using the spent mushroom substrate of Agaricus bisporus. Water 11(3):454
Marciniak M, Goscianska J, Frankowski M, Pietrzak R (2019) Optimal synthesis of oxidized mesoporous carbons for the adsorption of heavy metal ions. J Mol Liq 276:630–637. https://doi.org/10.1016/j.molliq.2018.12.042
Santos S C, Ungureanu G, Volf I, Boaventura R A, Botelho C M (2018) Macroalgae biomass as sorbent for metal ions. In Biomass as renewable raw material to obtain bioproducts of high-tech value (pp. 69–112). Elsevier. https://doi.org/10.1016/B978-0-444-63774-1.00003-X
Li K, Zheng Z, Li Y (2010) Characterization and lead adsorption properties of activated carbons prepared from cotton stalk by one-step H3PO4 activation. J Hazard Mater 181(1–3):440–447. https://doi.org/10.1016/j.jhazmat.2010.05.030
Jusoh A, Shiung LS, Noor MJMM (2007) A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 206(1–3):9–16. https://doi.org/10.1016/j.desal.2006.04.048
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33(11):2469–2479. https://doi.org/10.1016/S0043-1354(98)00475-8
Vinet L, Zhedanov AA (2010) Missing family of classical orthogonal polynomials. J (Can Dent Assoc) 70(3):156–157. https://doi.org/10.1520/D4607-14
Witek-Krowiak A, Chojnacka K, Podstawczyk D, Dawiec A, Pokomeda K (2014) Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Biores Technol 160:150–160. https://doi.org/10.1016/j.biortech.2014.01.021
Isam M, Baloo L, Kutty SRM, Yavari S (2019) Optimisation and modelling of Pb (II) and Cu (II) biosorption onto red algae (Gracilaria changii) by using response surface methodology. Water (Basel) 11(11):2325. https://doi.org/10.3390/w11112325
Xia Y, Yang T, Zhu N, Li D, Chen Z, Lang Q, Liu Z, Jiao W (2019) Enhanced adsorption of Pb (II) onto modified hydrochar: modeling and mechanism analysis. Biores Technol 288(April):1–8. https://doi.org/10.1016/j.biortech.2019.121593
Chowdhury ZZ (2013) Preparation, characterization and adsorption studies of heavy metals onto activated adsorbent materials derived from agricultural residues. Universiti Malaya, Jabatan Kimia, Fakulti Sains, Universiti Malaya, p 772
Hameed BH, Tan IAW, Ahmad AL (2008) Optimization of basic dye removal by oil palm fibre-based activated carbon using response surface methodology. J Hazard Mater 158(2–3):324–332. https://doi.org/10.1016/j.jhazmat.2008.01.088
Sutar SS, Patil PJ, Tamboli AS, Patil DN, Apine OA, Jadhav JP (2019) Biodegradation and detoxification of malachite green by a newly isolated bioluminescent bacterium Photobacterium leiognathi strain MS under RSM optimized culture conditions. Biocatalysis Agric Biotechnol 20:101183. https://doi.org/10.1016/j.bcab.2019.101183
Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163(2–3):650–656. https://doi.org/10.1016/j.jhazmat.2008.07.090
Mahmood T, Ali R, Naeem A, Hamayun M, Aslam M (2017) Potential of used Camellia sinensis leaves as precursor for activated carbon preparation by chemical activation with H3PO4; optimization using response surface methodology. Process Saf Environ Prot 109:548–563. https://doi.org/10.1016/j.psep.2017.04.024
Alslaibi TM, Abustan I, Azmier M, Abu A (2013) Journal of Environmental Chemical Engineering Cadmium removal from aqueous solution using microwaved olive stone activated carbon. Biochem Pharmacol 1(3):589–599. https://doi.org/10.1016/j.jece.2013.06.028
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. John Wiley & Sons
Lim WC, Srinivasakannan C, Balasubramanian N (2010) Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J Anal Appl Pyrol 88(2):181–186. https://doi.org/10.1016/j.jaap.2010.04.004
Guo J, Lua AC (2003) Textural and chemical properties of adsorbent prepared from palm shell by phosphoric acid activation. Mater Chem Phys 80(1):114–119. https://doi.org/10.1016/S0254-0584(02)00383-8
Bouhamed F, Elouear Z, Bouzid J (2012) Adsorptive removal of copper (II) from aqueous solutions on activated carbon prepared from Tunisian date stones: equilibrium, kinetics and thermodynamics. J Taiwan Inst Chem Eng 43(5):741–749. https://doi.org/10.1016/j.jtice.2012.02.011
Desa MNS, Ghani AZ, Talib SA, Tay CC (2016) Optimization of activated carbon preparation from spent mushroom farming waste (SMFW) via Box-Behnken design of response surface methodology. Malays J Nalytical Sci 20(3):461–468
Kumar A, Jena HM (2016) Preparation and characterization of high surface area activated carbon from fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys 6:651–658. https://doi.org/10.1016/j.rinp.2016.09.012
Aravindhan R, Raghava Rao J, Unni Nair B (2009) Preparation and characterization of activated carbon from marine macro-algal biomass. J Hazard Mater 162(2–3):688–694. https://doi.org/10.1016/j.jhazmat.2008.05.083
Salima A, Benaouda B, Noureddine B, Duclaux L (2013) Application of Ulva lactuca and Systoceira stricta algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Res 47(10):3375–3388. https://doi.org/10.1016/j.watres.2013.03.038
Nemchi F, Bestani B, Benderdouche N, Belhakem M, Duclaux L (2017) Enhancement of Ni2+ removal capacity of activated carbons obtained from Mediterranean Ulva lactuca and Systoceira stricta algal species. J Environ Chem Eng 5(3):2337–2345. https://doi.org/10.1016/j.jece.2017.03.027
Sulaiman NS, Hashim R, Mohamad Amini MH, Danish M, Sulaiman O (2018) Optimization of activated carbon preparation from cassava stem using response surface methodology on surface area and yield. J Clean Prod 198:1422–1430. https://doi.org/10.1016/j.jclepro.2018.07.061
Ronda A, Martín-Lara MA, Dionisio E, Blázquez G, Calero M (2013) Effect of lead in biosorption of copper by almond shell. J Taiwan Inst Chem Eng 44(3):466–473. https://doi.org/10.1016/j.jtice.2012.12.019
Hossain A (2013) Development of novel biosorbents in removing heavy metals from aqueous solution. University of Technology, Sydney (UTS)
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazard Mater 192(3):1827–1835. https://doi.org/10.1016/j.jhazmat.2011.07.019
Ibrahim WM, Hassan AF, Azab YA (2016) Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt J Basic Appl Sci 3(3):241–249. https://doi.org/10.1016/j.ejbas.2016.07.005
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2013) Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ Sci Pollut Res 20(5):3011–3023. https://doi.org/10.1007/s11356-012-1208-2
Foroutan R, Esmaeili H, Abbasi M, Rezakazemi M, Mesbah M (2018) Adsorption behavior of Cu (II) and Co (II) using chemically modified marine Algae. Environ Technol (UK) 39(21):2792–2800. https://doi.org/10.1080/09593330.2017.1365946
Van Thuan T, Quynh BTP, Nguyen TD, Ho VTT, Bach LG (2017) Response surface methodology approach for optimization of Cu2+, Ni2+and Pb2+adsorption using KOH-activated carbon from banana peel. Surfaces and Interfaces 6:209–217. https://doi.org/10.1016/j.surfin.2016.10.007
Zhai Y, Zeng G, Wang L, Wei X, Li C, Li S (2011) Removal of copper and lead ions from aqueous solutions by adsorbent derived from sewage sludge. Int J Environ Waste Manage 8(3–4):229–240
Goel J, Kadirvelu K, Rajagopal C, Garg VK (2005) Removal of lead (II) by adsorption using treated granular activated carbon: batch and column studies. J Hazard Mater 125(1–3):211–220. https://doi.org/10.1016/j.jhazmat.2005.05.032
Sekar M, Sakthi V, Rengaraj S (2004) Kinetics and equilibrium adsorption study of lead (II) onto activated carbon prepared from coconut shell. J Colloid Interface Sci 279(2):307–313
Lingamdinne LP, Koduru JR, Chang YY, Karri RR (2018) Process optimization and adsorption modeling of Pb (II) on nickel ferrite-reduced graphene oxide nano-composite. J Mol Liq 250:202–211. https://doi.org/10.1016/j.molliq.2017.11.174
Yavari S, Malakahmad A, Sapari NB (2016) Effects of production conditions on yield and physicochemical properties of biochars produced from rice husk and oil palm empty fruit bunches. Environ Sci Pollut Res 23(18):17928–17940. https://doi.org/10.1007/s11356-016-6943-3
Roy P, Dey U, Chattoraj S, Mukhopadhyay D, Mondal NK (2017) Modeling of the adsorptive removal of arsenic (III) using plant biomass: a bioremedial approach. Appl Water Sci 7(3):1307–1321. https://doi.org/10.1007/s13201-015-0339-2
Mondal NK, Samanta A, Roy P, Das B (2019) Optimization study of adsorption parameters for removal of Cr (VI) using Magnolia leaf biomass by response surface methodology. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-019-00322-5
Boudrahem F, Soualah A, Aissani-Benissad F (2011) Pb (II) and Cd (II) removal from aqueous solutions using activated carbon developed from coffee residue activated with phosphoric acid and zinc chloride. J Chem Eng Data 56(5):1946–1955
Astuti W, Chafidz A, Al-Fatesh AS, Fakeeha AH (2021) Removal of lead (Pb (II)) and zinc (Zn (II)) from aqueous solution using coal fly ash (CFA) as a dual-sites adsorbent. Chin J Chem Eng 34:289–298
Esmaeili A, Ghasemi S, Rustaiyan A (2008). Evaluation of the activated carbon prepared from the algae Gracilaria for the biosorption of Cu (II) from aqueous solutions. Afr J Biotechnol 7(12). https://doi.org/10.4314/ajb.v7i12.58884
Romera E, González F, Ballester A, Blázquez ML, Munoz JA (2007) Comparative study of biosorption of heavy metals using different types of algae. Biores Technol 98(17):3344–3353. https://doi.org/10.1016/j.biortech.2006.09.026
Lee YC, Chang SP (2011) The biosorption of heavy metals from aqueous solution by Spirogyra and Cladophora filamentous macroalgae. Biores Technol 102(9):5297–5304. https://doi.org/10.1016/j.biortech.2010.12.103
Mehta SK, Gaur JP (2001) Characterization and optimization of Ni and Cu sorption from aqueous solution by Chlorella vulgaris. Ecol Eng 18(1):1–13
Corral-Bobadilla M, Lostado-Lorza R, Somovilla-Gómez F, Escribano-García R (2022) Biosorption of Cu (II) ions as a method for the effective use of activated carbon from grape stalk waste: RMS optimization and kinetic studies. Energy Sour, Part A: Recov Util Environ Effects 44(2):4706–4726
Peng SH, Wang R, Yang LZ, He L, He X, Liu X (2018) Biosorption of copper, zinc, cadmium and chromium ions from aqueous solution by natural foxtail millet shell. Ecotoxicol Environ Saf 165:61–69. https://doi.org/10.1016/j.ecoenv.2018.08.084
Oliveira RC, Hammer P, Guibal E, Taulemesse JM, Garcia O (2014) Characterization of metal-biomass interactions in the lanthanum (III) biosorption on Sargassum sp. using SEM/EDX, FTIR, and XPS: preliminary studies. Chem Eng J 239:381–391. https://doi.org/10.1016/j.cej.2013.11.042
Yuan W, Cheng J, Huang H, Xiong S, Gao J, Zhang J, Feng S (2019) Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol Environ Saf 175:138–147. https://doi.org/10.1016/j.ecoenv.2019.03.057
do Nascimento JM, de Oliveira JD, Rizzo ACL (2018) Leite SGF (2019) Biosorption Cu (II) by the yeast Saccharomyces cerevisiae. Biotechnol Rep 21:e00315. https://doi.org/10.1016/j.btre.2019.e00315
Sarı A, Tuzen M, Uluözlü ÖD, Soylak M (2007) Biosorption of Pb (II) and Ni (II) from aqueous solution by lichen (Cladonia furcata) biomass. Biochem Eng J 37(2):151–158. https://doi.org/10.1016/j.bej.2007.04.007
Daneshvar E, Vazirzadeh A, Niazi A, Sillanpää M, Bhatnagar A (2017) A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae – effect of pretreatment. Chem Eng J 307:435–446. https://doi.org/10.1016/j.cej.2016.08.093
Li Y, Song S, Xia L, Yin H, García Meza JV, Ju W (2019) Enhanced Pb (II) Removal by algal-based biosorbent cultivated in high-phosphorus cultures. Chem Eng J 361:167–179. https://doi.org/10.1016/j.cej.2018.12.070
Coates J (2006) Interpretation of infrared spectra, a practical approach. Encycl Anal Chem: Appl Theory Instrum
El Azm NA, Fleita D, Rifaat D, Mpingirika EZ, Amleh A, El-Sayed MMH (2019) Production of bioactive compounds from the sulfated polysaccharides extracts of Ulva lactuca: post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules 24(11):1–17. https://doi.org/10.3390/molecules24112132
Cardoso I, Cotas J, Rodrigues A, Ferreira D, Osório N, Pereira L (2019) Extraction and analysis of compounds with antibacterial potential from the Red Alga Grateloupia Turuturu. J Marine Sci Eng 7(7):220. https://doi.org/10.3390/jmse7070220
Pavasant P, Apiratikul R, Sungkhum V, Suthiparinyanont P, Wattanachira S, Marhaba TF (2006) Biosorption of Cu2+, Cd2+, Pb2+, and Zn2+ using dried marine green macroalga Caulerpa lentillifera. Biores Technol 97(18):2321–2329. https://doi.org/10.1016/j.biortech.2005.10.032
Rangabhashiyam S, Balasubramanian P (2018) Characteristics, performances, equilibrium and kinetic modeling aspects of heavy metal removal using algae. Bioresource Technol Rep 5:261–279. https://doi.org/10.1016/j.biteb.2018.07.009
Momčilović M, Purenović M, Bojić A, Zarubica A, Randelovid M, Ranđelović M (2011) Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 276(1–3):53–59. https://doi.org/10.1016/j.desal.2011.03.013
Acknowledgements
The authors sincerely appreciate the Universiti Teknologi PETRONAS, Fishery Department of Langkawi, and Al-Mustaqbal University College for their strong support in this study.
Funding
Open access funding provided by Lulea University of Technology. This research was funded by the STIRF (grant number 0153AA-D76), YUTP (grant number 0153AA-H32), Universiti Teknologi PETRONAS, Malaysia, and Al-Mustaqbal University College (MUC) (grant number MUC-E-0122).
Author information
Authors and Affiliations
Contributions
Conceptualization: Mubeen Isam, Lavania Baloo. Methodology: Mubeen Isam, Lavania Baloo. Formal analysis: Mubeen Isam, Lavania Baloo. Writing—original draft preparation: Mubeen Isam, Lavania Baloo. Writing—review and editing: Mubeen Isam, Lavania Baloo, Ali Chabuk, Ali Majdi, Nadhir Al-Ansari. Funding acquisition: Lavania Baloo, Nadhir Al-Ansari, Ali Majdi. Supervision: Lavania Baloo, Nadhir Al-Ansari.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isam, M., Baloo, L., Chabuk, A. et al. Optimization and modelling of Pb (II) and Cu (II) adsorption onto red algae (Gracilaria changii)-based activated carbon by using response surface methodology. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04150-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04150-8