Abstract
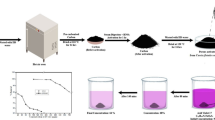
The application of agriculture waste–derived activated carbon for the removal of organic pollutants from wastewater is an interesting approach for the environmental security. In this research study, soya bean residuals and cotton seeds were utilized for the preparation of an activated carbon material as a valuable adsorbent for the elimination of dye molecules during wastewater treatment. The novelty of this study is based on the utilization of such agro-mixture, as first time, for the production of activated carbon species. The characteristics of the produced activated carbon were determined using XRD, SEM, FTIR, BET surface area, and Raman spectroscopy analyses. The resulting carbonaceous material was used as an adsorbent material for the removal of anionic and cationic dyes in aqueous solutions. Kinetic investigation could reveal that the elimination process of methyl orange (MO) and crystal violet (CV) reached equilibrium at 120 min and the uptake reaction obeyed to the pseudo-second-order kinetic model. The analysis of the sorption results using several isotherm models shows that the Langmuir isotherm model is best fitting for MO and CV sorption process. The maximum sorption capacity for both dyes was found to be 131.5 and 57.1 mg g−1 for MO and CV respectively. These findings could reflect the high affinity of the prepared activated carbon toward the anionic dye over the cationic one. The thermodynamic parameters exhibit that the uptake of anionic as well as cationic dyes is an endothermic, feasible, and spontaneous process. Obtained results through this research work are revealing great significance for environmental implications based on usage of solid waste residuals for obtaining efficient carbon material as a promising adsorbent for organic pollutants from waste aqueous solutions.










Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gomaa H, Sayed A, Mahross MH, Abdel-Hakim M, Othman IM, Li J, El-Bahy SM (2022) A hybrid spongy-like porous carbon-based on azopyrazole-benzenesulfonamide derivative for highly selective Fe3+-adsorption from real water samples. Microporous Mesoporous Mater 1(330):111578
Islam A, Teo SH, Taufiq-Yap YH, Ng CH, Vo DV, Ibrahim ML, Hasan MM, Khan MA, Nur AS, Awual MR (2021) Step towards the sustainable toxic dyes removal and recycling from aqueous solution-a comprehensive review. Resour Conserv Recycl 1(175):105849
Teo SH, Ng CH, Islam A, Abdulkareem-Alsultan G, Joseph CG, Janaun J, Taufiq-Yap YH, Khandaker S, Islam GJ, Znad H, Awual MR (2022) Sustainable toxic dyes removal with advanced materials for clean water production: a comprehensive review. J Clean Prod 15(332):130039
Kumar M, Tamilarasan R (2013) Modeling of experimental data for the adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Prosopis juliflora. Polish J Chem Technol 15:29–39
Gong R, Ye J, Dai W, Yan X, Hu J, Hu X, Li S, Huang H (2013) Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind Eng Chem Res 52:14297–14303
Huang X, Zhan X, Wen C, Xu F, Luo L (2018) Amino-functionalized magnetic bacterial cellulose/activated carbon composite for Pb2+ and methyl orange sorption from aqueous solution. J Mater Sci Technol 34:855–863
Salem MA, Bakr EA, El-Attar HG (2018) Pt@Ag and Pd@Ag core/shell nanoparticles for catalytic degradation of Congo Red in aqueous solution. Spectrochim. Acta Part A Mol Biomol Spectrosc 188:155–163
Zhang G, Wu J, Zeng H, Neary MC, Devany M, Zheng S, Dub PA (2019) Dearomatization and functionalization of terpyridine ligands leading to unprecedented zwitterionic meisenheimer aluminum complexes and their use in catalytic hydroboration. ACS Catal 9:874–884
Kassem KO, Hussein MA, Motawea MM, Gomaa H, Alrowaili ZA, Ezzeldien M (2021) Design of mesoporous ZnO@ silica fume-derived SiO2 nanocomposite as photocatalyst for efficient crystal violet removal: effective route to recycle industrial waste. J Clean Prod 1(326):129416
Gomaa H, Hussein MA, Motawea MM, Aboraia AM, Cheira MF, Alotaibi MT, El-Bahy SM, Ali HM (2022) A hybrid mesoporous CuO@ barley straw-derived SiO2 nanocomposite for adsorption and photocatalytic degradation of methylene blue from real wastewater. Colloids Surf, A 5(644):128811
Hasan MM, Shenashen MA, Hasan MN, Znad H, Salman MS, Awual MR (2021) Natural biodegradable polymeric bioadsorbents for efficient cationic dye encapsulation from wastewater. J Mol Liq 1(323):114587
Munjur HM, Hasan MN, Awual MR, Islam MM, Shenashen MA, Iqbal J (2020) Biodegradable natural carbohydrate polymeric sustainable adsorbents for efficient toxic dye removal from wastewater. J Mol Liq 1(319):114356
Wang X, Xu Q, Zhang L, Pei L, Xue H, Li Z (2022) Adsorption of methylene blue and Congo Red from aqueous solution on 3D MXene/carbon foam hybrid aerogels: a study by experimental and statistical physics modeling. J Environ Chem Eng 22:109206
AbdEl-Salam AH, Ewais HA, Basaleh AS (2017) Silver nanoparticles immobilised on the activated carbon as efficient adsorbent for removal of crystal violet dye from aqueous solutions A kinetic study. J Mol Liq 248:833–841
Loulidi I, Boukhlifi F, Ouchabi M, Amar A, Jabri M, Kali A, Chraibi S, Hadey C, Aziz F (2020) Adsorption of crystal violet onto an agricultural waste residue: kinetics, isotherm, thermodynamics, and mechanism of adsorption. Sci World J 2020:1. https://doi.org/10.1155/2020/5873521
Rai P, Gautam RK, Banerjee S, Rawat V, Chattopadhyaya MC (2015) Synthesis and characterization of a novel SnFe2O4@activated carbon magnetic nanocomposite and its effectiveness in the removal of crystal violet from aqueous solution. J Environ Chem Eng 3:2281–2291
Mane VS, Babu PVV (2011) Studies on the adsorption of Brilliant Green dye from aqueous solution onto low-cost NaOH treated saw dust. Desalination 273:321–329
Naushad M, Alqadami AA, Al-Kahtani AA, Ahamad T, Awual MR, Tatarchuk T (2019) Adsorption of textile dye using para-aminobenzoic acid modified activated carbon: kinetic and equilibrium studies. J Mol Liq 15(296):112075
Gomaa H, El-Monaem A, Eman M, Eltaweil AS, Omer AM (2022) Efficient removal of noxious methylene blue and crystal violet dyes at neutral conditions by reusable montmorillonite/NiFe2O4@ amine-functionalized chitosan composite. Sci Rep 12(1):1–6
Errais E, Duplay J, Darragi F, M’Rabet I, Aubert A, Huber F, Morvan G (2011) Efficient anionic dye adsorption on natural untreated clay: kinetic study and thermodynamic parameters. Desalination 275:74–81
Li Z, Hanafy H, Zhang L, Sellaoui L, Netto MS, Oliveira ML, Seliem MK, Dotto GL, Bonilla-Petriciolet A, Li Q (2020) Adsorption of Congo Red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: experiments, characterization and physical interpretations. Chem Eng J 15(388):124263
Ramutshatsha-Makhwedzha D, Mavhungu A, Moropeng ML, Mbaya R (2022) Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 8(8):e09930
Hussein MA, Motawea MM, Elsenety MM, El-Bahy SM, Gomaa H (2022) Mesoporous spongy Ni–Co oxides@ wheat straw-derived SiO2 for adsorption and photocatalytic degradation of methylene blue pollutants. Appl Nanosci 12(5):1519–1536
Su Y, Zhao B, Xiao W, Han R (2013) Adsorption behavior of light green anionic dye using cationic surfactant-modified wheat straw in batch and column mode. Environ Sci Pollut Res 20:5558–5568
Morshedy AS, Taha MH, El-Aty DMA, Bakry A, El Naggar AMA (2021) Solid waste sub-driven acidic mesoporous activated carbon structures for efficient uranium capture through the treatment of industrial phosphoric acid. Environ Technol Innov 21:101363
Masoud AM (2020) Sorption behavior of uranium from sulfate media using Purolite A400 as a strong base anion exchange resin. Int J Environ Anal Chem 102:3124–3146
Taha MH, Abdel Maksoud SA, Ali MM, El Naggar AMA, Morshedy AS, Elzoghby AA (2019) Conversion of biomass residual to acid-modified bio-chars for efficient adsorption of organic pollutants from industrial phosphoric acid: an experimental, kinetic and thermodynamic study. Int J Environ Anal Chem 99:1211–1234
Largitte L, Pasquier R (2016) A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des 109:495–504
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Xue H, Wang X, Xu Q, Dhaouadi F, Sellaoui L, Seliem MK, Lamine AB, Belmabrouk H, Bajahzar A, Bonilla-Petriciolet A, Li Z (2022) Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: a comparative study by experimental and advanced modeling analysis. Chem Eng J 15(430):132801
Xue H, Gao X, Seliem MK, Mobarak M, Dong R, Wang X, Fu K, Li Q, Li Z (2023) Efficient adsorption of anionic azo dyes on porous heterostructured MXene/biomass activated carbon composites: experiments, characterization, and theoretical analysis via advanced statistical physics models. Chem Eng J 1(451):138735
Kubra KT, Salman MS, Hasan MN (2021) Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J Mol Liq 15(328):115468
Kubra KT, Salman MS, Znad H, Hasan MN (2021) Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J Mol Liq 1(329):115541
Bhat VS, Kanagavalli P, Sriram G, Rampya PB, John NS, Veerapandian M, Kurkuri M, Hegde G (2020) Low cost, catalyst free, high performance supercapacitors based on porous nano carbon derived from agriculture waste. J. Energy Storage 32:101829
Ma J, Yu F, Zhou L, Jin L, Yang M, Luan J, Tang Y, Fan H, Yuan Z, Chen J (2012) Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl Mater Interfaces 4:5749–5760
Aravindhan R, Fathima NN, Rao JR, Nair BU (2007) Equilibrium and thermodynamic studies on the removal of basic black dye using calcium alginate beads. Colloids Surfaces A Physicochem Eng Asp 299:232–238
Pan B, Xing B (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42:9005–9013
El Naggar AMA, Ali MM, Abdel Maksoud SA, Taha MH, Morshedy AS, Elzoghby AA (2019) Waste generated bio-char supported co-nanoparticles of nickel and cobalt oxides for efficient adsorption of uranium and organic pollutants from industrial phosphoric acid. J Radioanal Nucl Chem 320:741–755
Younes AA, Masoud AM, Taha MH (2021) Amino-functionalised cross-linked polyacrylamide for the adsorption of U(VI) ions from contaminated aqueous solutions. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2021.2003348
Haddadian Z, Shavandi MA, Abidin Z, Fakhru’l-Razi A, Halim M, Ismail S (2013) Removal methyl orange from aqueous solutions using dragon fruit (Hylocereus undatus) foliage. Chem Sci Trans 2:900–910
Hasan MN, Shenashen MA, Hasan MM, Znad H, Awual MR (2021) Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent. Chemosphere 1(270):128668
Younes AA, Masoud AM, Taha MH (2018) Uranium sorption from aqueous solutions using polyacrylamide-based chelating sorbents. Sep Sci Technol 53:2573–2586
Hassanein TF, Masoud AM, Mohamed WS, Taha MH, Guibal E (2021) Synthesis of polyamide 6/nano-hydroxyapatite hybrid (PA6/n-HAp) for the sorption of rare earth elements and uranium. J Environ Chem Eng 9:104731
Su Y, Jiao Y, Dou C, Han R (2014) Biosorption of methyl orange from aqueous solutions using cationic surfactant-modified wheat straw in batch mode. Desalin Water Treat 52:6145–6155
Xiao Y, Hill JM (2018) Benefit of hydrophilicity for adsorption of methyl orange and electro-Fenton regeneration of activated carbon-polytetrafluoroethylene electrodes. Environ Sci Technol 52:11760–11768
Gapusan RB, Balela MDL (2020) Adsorption of anionic methyl orange dye and lead(II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater Chem Phys 243:122682
Sulyman M, Namiesnik J, Gierak A (2014) Utilization of new activated carbon derived from oak leaves for removal of crystal violet from aqueous solution. Polish J Environ Stud 23:2223–2232
Hamidzadeh S, Torabbeigi M, Shahtaheri SJ (2015) Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J Environ Heal Sci Eng 13:1–7
Porkodi K, Vasanth Kumar K (2007) Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater 143:311–327
Yeamin MB, Islam MM, Chowdhury AN, Awual MR (2021) Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J Clean Prod 1(291):125920
Silva VC, Araújo MEB, Rodrigues AM, Vitorino MDBC, Cartaxo JM, Menezes RR, Neves GA (2021) Adsorption behavior of crystal violet and Congo Red dyes on heat-treated Brazilian palygorskite: kinetic, isothermal and thermodynamic studies. Mater 14:5688
Jia Y, Ding L, Ren P, Zhong M, Ma J, Fan X (2020) Performances and mechanism of methyl orange and Congo Red adsorbed on the magnetic ion-exchange resin. J Chem Eng Data 65:725–736
Haque E, Jun JW, Jhung SH (2011) Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J Hazard Mater 185:507–511
Yu F, Chen J, Chen L, Huai J, Gong W, Yuan Z, Wang J, Ma J (2012) Magnetic carbon nanotubes synthesis by Fenton’s reagent method and their potential application for removal of azo dye from aqueous solution. J Colloid Interface Sci 378:175–183
Yao Y, Xu F, Chen M, Xu Z, Zhu Z (2010) Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol 101:3040–3046
Jalil AA, Triwahyono S, Adam SH, Rahim ND, Aziz MAA, Hairom NHH, Razali NAM, Abidin MAZ, Mohamadiah MKA (2010) Adsorption of methyl orange from aqueous solution onto calcined Lapindo volcanic mud. J Hazard Mater 181:755–762
Nizamov SN, Barakaeva MN, Kurtaliev EN, Tatarets AL, Patsenker LD (2009) Influence of molecular structure of squaraine dyes on their aggregation in aqueous solutions. J Appl Spectrosc 76:464–469
Acknowledgements
The authors extend their appreciation to the Scientific Council at King Khalid University for funding this work.
Author information
Authors and Affiliations
Contributions
Ahmed Masoud: experimental operation, data interpretation, writing – reviewing and editing. Asmaa Zahran: Synthesis, data curation and interpretation, writing – original draft. Adel El-Zahhar: methodology, validation, data curation and interpretation, writing – original draft, reviewing and editing. Hoda Ahmed: synthesis, data curation and interpretation. Majed Ghamdi: methodology, validation, data curation and interpretation, writing – original draft. Ahmed El Naggar: synthesis, characterization, data curation and interpretation, writing – original draft, reviewing and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zahran, A.I., El-Zahhar, A.A., Ahmed, H.S. et al. Mixture of soya bean and cotton seed residuals for production of activated carbon species as efficient adsorbent in the process of wastewater decontamination via dye disposal. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03941-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03941-3