Abstract
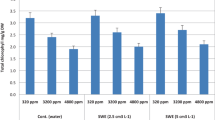
Temperature change, global warming, and the ever-changing climate have made life extremely difficult for plants. Our research analyzed the effects of chaste plant extract on salt-stressed tomatoes. Three concentrations of chaste plant extract (CPE) were applied (0.25%, 0.5%, and 1%). The obtained results indicated that CPE-treated plants showed a higher ability to tolerate salt stress (75 mM) by a significant increase in plant growth and photosynthetic pigment content (p ≤ 0.05). Antioxidant enzyme activities such as superoxide dismutase (SOD), isocitrate dehydrogenase (ICDH), glutathione peroxidase (GPx), and glutathione reductase (GR) are also crucial in the case of treatments. Carbon–nitrogen enzymes such as phosphoenolpyruvate carboxylase (PEPC), malate dehydrogenase (NAD-MDH), and glutamine synthase have changed (GS). Our results suggest that the application of chaste plant extract could be used as a promising plant growth biostimulant for treating tomato plants under salinity stress.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are included in the article.
References
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. CRC Crit Rev Plant Sci 32:237–249. https://doi.org/10.1080/07352689.2013.758544
Acosta-Motos JR et al (2015) NaCl-induced physiological and biochemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J Plant Physiol 183:41–51. https://doi.org/10.1016/j.jplph.2015.05.005
Misra SC et al (2006) Relationship between carbon isotope discrimination, ash content and grain yield in wheat in the Peninsular Zone of India. J Agron Crop Sci 192:352–362. https://doi.org/10.1111/j.1439-037X.2006.00225.x
Abd El-Baky HH, El-Baroty GS (2012) Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct 3:381–388. https://doi.org/10.1039/c2fo10194g
Kiddle G et al (2003) Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxidants Redox Signal 5:23–32. https://doi.org/10.1089/152308603321223513
Salim BBM (2016) Influence of biochar and seaweed extract applications on growth, yield and mineral composition of wheat (Triticum aestivum L.) under sandy soil conditions. Ann Agric Sci 61:257–265. https://doi.org/10.1016/j.aoas.2016.06.001
Ono M et al (2011) A new diterpenoid glucoside and two new diterpenoids from the fruit of Vitex agnus-castus. Chem Pharm Bull 59:392–396. https://doi.org/10.1248/cpb.59.392
Ben Mrid R, et al (2021) Secondary metabolites as biostimulant and bioprotectant agents: a review. Sci Total Environ 777. https://doi.org/10.1016/j.scitotenv.2021.146204
Bouchmaa N, et al (2022) Beta vulgaris subsp. maritima: a valuable food with high added health benefits. mdpi.com
Naboulsi I, et al (2022) Crataegus oxyacantha extract as a biostimulant to enhance tolerance to salinity in tomato plants. Plants 11. https://doi.org/10.3390/plants11101283
Ennoury A et al (2022) River’s Ulva intestinalis extract protects common bean plants (Phaseolus vulgaris L.) against salt stress. South African J Bot 150:334–341. https://doi.org/10.1016/j.sajb.2022.07.035
Ben Mrid R, et al (2019) Phytochemical Characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus subsp. Oxycedrus needles and berries. Molecules 24. https://doi.org/10.3390/molecules24030502
Kubiś J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ehmann A (1977) The van URK-Salkowski reagent - a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr A 132:267–276. https://doi.org/10.1016/S0021-9673(00)89300-0
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Roussi Z et al (2022) Insight into Cistus salviifolius extract for potential biostimulant effects in modulating cadmium-induced stress in sorghum plant. Physiol Mol Biol Plants 28:1323–1334. https://doi.org/10.1007/s12298-022-01202-7
Armstrong FH, et al (1947) The estimation of carbohydrates in plant extracts by anthrone. ncbi.nlm.nih.gov 30:700
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Bouchmaa N, et al (2018) Cytotoxicity of new pyridazin-3(2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch Pharm (Weinheim) 351. https://doi.org/10.1002/ardp.201800128
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136. https://doi.org/10.1104/pp.110.1.125
Ben Mrid R et al (2018) Activities of carbon and nitrogen metabolism enzymes of sorghum (Sorghum bicolor L. Moench) during seed development. J Crop Sci Biotechnol 21:283–289. https://doi.org/10.1007/s12892-017-0140-0
El Omari R, Ben Mrid R, Chibi F, Nhiri M (2016) Involvement of phosphoenolpyruvate carboxylase and antioxydants enzymes in nitrogen nutrition tolerance in Sorghum bicolor plants. Russ J Plant Physiol 63:719–726. https://doi.org/10.1134/S102144371606008X
Kchikich A et al (2021) Effect of arbuscular mycorrhizal fungus and the γ-aminobutyric acid treatment in nitrate assimilation under nitrogen deficiency in sorghum plant. Russ J Plant Physiol 68:901–908. https://doi.org/10.1134/S102144372105006X
Ben Mrid R et al (2016) Effect of nitrogen source and concentration on growth and activity of nitrogen assimilation enzymes in roots of a moroccan sorghum ecotype. Plant 4:71. https://doi.org/10.11648/j.plant.20160406.14
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Latique S, et al (2021) Foliar application of Ulva rigida water extracts improves salinity tolerance in wheat (Triticum durum l.). Agronomy 11. https://doi.org/10.3390/agronomy11020265
Bulgari R, Franzoni G, Ferrante A (2019) Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 9. https://doi.org/10.3390/agronomy9060306
Claros Cuadrado JL, et al (2019) Insecticidal properties of capsaicinoids and glucosinolates extracted from capsicum chinense and tropaeolum tuberosum. Insects 10. https://doi.org/10.3390/insects10050132
Egamberdieva D (2009) Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol Plant 31:861–864. https://doi.org/10.1007/s11738-009-0297-0
Cetinkaya H et al (2017) Flavonoid accumulation behavior in response to the abiotic stress: can a uniform mechanism be illustrated for all plants? Flavonoids - From Biosynth to Hum Heal. https://doi.org/10.5772/68093
Fawzy Z, Abd El-Baky M, Mahmoud R (2010) Response of snap bean plants to mineral fertilizers and humic acid application. Res J Agric Biol Sci 6:169–175
Duman F, Ozturk F, Aydin Z (2010) Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): effects of concentration and duration of exposure. Ecotoxicology 19:983–993. https://doi.org/10.1007/s10646-010-0480-5
Rizwan M et al (2017) Foliar application of aspartic acid lowers cadmium uptake and Cd-induced oxidative stress in rice under Cd stress. Environ Sci Pollut Res 24:21938–21947. https://doi.org/10.1007/s11356-017-9860-1
Bhuyan MHMB, et al (2020) Modulation of cadmium tolerance in rice: insight into vanillic acid-induced upregulation of antioxidant defense and glyoxalase systems. Plants 9. https://doi.org/10.3390/plants9020188
Hamilton EW, Heckathorn SA (2001) Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas Complex II is protected by proline and betaine. Plant Physiol 126:1266–1274. https://doi.org/10.1104/pp.126.3.1266
Hoque MA et al (2008) Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J Plant Physiol 165:813–824. https://doi.org/10.1016/j.jplph.2007.07.013
Faraz A et al (2020) Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regul 39:641–655. https://doi.org/10.1007/s00344-019-10007-0
Mallick N, Mohn FH (2000) Reactive oxygen species: response of algal cells. J Plant Physiol 157:183–193. https://doi.org/10.1016/S0176-1617(00)80189-3
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76. https://doi.org/10.1016/S0098-8472(02)00058-8
Marín-Hernández Á, et al (2016) Inhibition of non-flux-controlling enzymes deters cancer glycolysis by accumulation of regulatory metabolites of controlling steps. Front Physiol 7. https://doi.org/10.3389/fphys.2016.00412
Ozfidan-Konakci C, Yildiztugay E, Kucukoduk M (2015) Upregulation of antioxidant enzymes by exogenous gallic acid contributes to the amelioration in Oryza sativa roots exposed to salt and osmotic stress. Environ Sci Pollut Res 22:1487–1498. https://doi.org/10.1007/s11356-014-3472-9
Nasri N et al (2011) Chemical compounds from Phoenician juniper berries (Juniperus phoenicea). Nat Prod Res 25:1733–1742. https://doi.org/10.1080/14786419.2010.523827
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Sengupta S, Majumder AL (2009) Insight into the salt tolerance factors of a wild halophytic rice, Porteresia coarctata: a physiological and proteomic approach. Planta 229:911–929. https://doi.org/10.1007/s00425-008-0878-y
Sathish GKC (2017) Effect of salinity stress on carbohydrate, lipid peroxidation and proline contents of two horse gram [Macrotyloma uniflorum (Lam.) Verdc] varieties. J Sci Agric 1:37. https://doi.org/10.25081/jsa.2017.v1i0.30
Ben Mansour R, Ben Fattoum R, Gouia H, Haouari CC (2019) Salt stress effects on enzymes activities involved in carbon metabolism and nitrogen availability of two Tunisian durum wheat varieties. J Plant Nutr 42:1142–1151. https://doi.org/10.1080/01904167.2019.1604749
Jeanneau M et al (2002) Manipulating PEPC levels in plants. J Exp Bot 53:1837–1845. https://doi.org/10.1093/jxb/erf061
Qin N, Xu W, Hu L et al (2016) Drought tolerance and proteomics studies of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene. Protoplasma 253:1503–1512. https://doi.org/10.1007/s00709-015-0906-2
Kumar S, Tsai CJ, Nussinov R (2000) Factors enhancing protein thermostability. Protein Eng 13:179–191. https://doi.org/10.1093/protein/13.3.179
Surabhi GK, Reddy AM, Kumari GJ, Sudhakar C (2008) Modulations in key enzymes of nitrogen metabolism in two high yielding genotypes of mulberry (Morus alba L.) with differential sensitivity to salt stress. Environ Exp Bot 64:171–179. https://doi.org/10.1016/j.envexpbot.2008.04.006
Skopelitis DS et al (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18:2767–2781. https://doi.org/10.1105/tpc.105.038323
Wang H, Zhang M, et al (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol 12. https://doi.org/10.1186/1471-2229-12-194
Shi J et al (2015) Phosphoenolpyruvate carboxylase in Arabidopsis leaves plays a crucial role in carbon and nitrogen metabolism. Plant Physiol 167:671–681. https://doi.org/10.1104/pp.114.254474
Author information
Authors and Affiliations
Contributions
Conceptualization, Ennoury Abdelhamid; methodology, Ennoury Abdelhamid, Roussi Zoulfa, Nada Nhhala, Zouaoui Zakia, Krid Azzouz, Kchikich Anass, and Kabach Imad; formal analysis and investigation, Nhiri Mohamed; writing—original draft preparation, Ennoury Abdelhamid and Roussi Zoulfa; writing—review and editing, Ennoury Abdelhamid and Nhiri Mohamed; supervision, Nhiri Mohamed.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelhamid, E., Zoulfa, R., Nada, N. et al. Chaste plant extract is a promising biostimulant for tomato plants’ growth under salt stress. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03454-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03454-5