Abstract
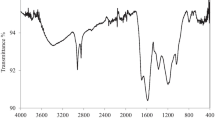
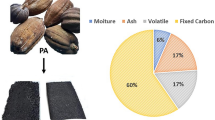
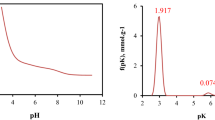
In this study, novel biochar was fabricated from pumpkin peels with phosphoric acid activation (PPBPA) and characterized by ultimate and proximate analysis, BET surface area determination, SEM, FT-IR spectroscopy and pHPZC assay. The PPBPA demonstrated quite a high surface area, microporous structure and low ash content with values of 689.9 m2/g, 6.0 Å, and 6.4%, respectively. The PPBPA was examined for the batch removal of ciprofloxacin from aqueous solutions by investigating parameters such as pH, contact time, PPBPA dosage and initial ciprofloxacin (CPX) concentration. The removal of CPX was maximum at a pH of 8.0 and reached equilibrium at 24 h. The CPX adsorption onto PPBPA showed high agreement with the pseudo-second-order kinetic model. The percent removal of CPX decreased with the increasing initial concentration of CPX. The CPX adsorption equilibrium data onto PPBPA were found to be compatible with both Freundlich and Langmuir isotherm models. The maximum CPX adsorption capacity of PPBPA was calculated to be 153.9 mg/g from the Langmuir equation. CPX adsorption increased with increasing temperature, indicating that the adsorption was endothermic. Adsorption/desorption experiment results showed that CPX desorption efficiency was higher within 0.1 M NaOH than 0.1 M HCl. As a result, this study revealed that PPBPA is an underutilized bioresource with the potential to provide an excellent, low-cost, easy-to-prepare adsorbent for CPX removal in wastewater treatment.








Similar content being viewed by others
References
Nas B, Dolu T, Koyuncu S (2021) Behavior and Removal of Ciprofloxacin and Sulfamethoxazole Antibiotics in Three Different Types of Full-Scale Wastewater Treatment Plants: A Comparative Study. Water Air Soil Pollut 232:1–15. https://doi.org/10.1007/S11270-021-05067-6
Larsson DGJ (2014) Antibiotics in the environment. Ups J Med Sci 119:108–112. https://doi.org/10.3109/03009734.2014.896438
Basturk I, Varank G, Murat-Hocaoglu S et al (2021) Simultaneous degradation of cephalexin, ciprofloxacin, and clarithromycin from medical laboratory wastewater by electro-Fenton process. J Environ Chem Eng 9:104666. https://doi.org/10.1016/j.jece.2020.104666
Huang W, Chen J, Zhang J (2020) Removal of ciprofloxacin from aqueous solution by rabbit manure biochar. Environ Technol 41:1380–1390. https://doi.org/10.1080/09593330.2018.1535628
Chen J, Ouyang J, Cai X et al (2021) Removal of ciprofloxacin from water by millimeter-sized sodium alginate/H3PO4 activated corncob-based biochar composite beads. Sep Purif Technol 276:119371. https://doi.org/10.1016/J.SEPPUR.2021.119371
Kong X, Liu Y, Pi J et al (2017) Low-cost magnetic herbal biochar: characterization and application for antibiotic removal. Environ Sci Pollut Res 24:6679–6687. https://doi.org/10.1007/s11356-017-8376-z
Mohan S, Balakrishnan P (2021) Kinetics of ciprofloxacin removal using a sequential two-step ozonation-biotreatment process. Environ Technol Innov 21:101284. https://doi.org/10.1016/J.ETI.2020.101284
Gupta B, Gupta AK, Tiwary CS, Ghosal PS (2021) A multivariate modeling and experimental realization of photocatalytic system of engineered S-C3N4/ZnO hybrid for ciprofloxacin removal: Influencing factors and degradation pathways. Environ Res 196:110390. https://doi.org/10.1016/j.envres.2020.110390
An T, Yang H, Li G et al (2010) Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl Catal B Environ 94:288–294. https://doi.org/10.1016/J.APCATB.2009.12.002
Bhattacharya P, Mukherjee D, Dey S et al (2019) Development and performance evaluation of a novel CuO/TiO 2 ceramic ultrafiltration membrane for ciprofloxacin removal. Mater Chem Phys J 229:106–116. https://doi.org/10.1016/j.matchemphys.2019.02.094
Kaya AE, Deveci H, Martínez-Máñez R (2021) Low-cost silica xerogels as potential adsorbents for ciprofloxacin removal. Sustain Chem Pharm 22:100483. https://doi.org/10.1016/J.SCP.2021.100483
Ghani AA, Shahzad A, Moztahida M et al (2021) Adsorption and electrochemical regeneration of intercalated Ti3C2Tx MXene for the removal of ciprofloxacin from wastewater | Elsevier Enhanced Reader. Chem Eng J 421:127780. https://doi.org/10.1016/j.cej.2020.127780
Ozer C (2020) Kinetic and equilibrium studies on the batch removal of methylene blue from aqueous solution by using natural magnetic sand. Desalin Water Treat 201:393–403. https://doi.org/10.5004/dwt.2020.26204
Li J, Yu G, Pan L et al (2020) Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ Sci Pollut Res 27:22806–22817. https://doi.org/10.1007/S11356-020-08333-Y
Soylak M, Doğan M (1996) Column preconcentration of trace amounts of copper on activated carbon from natural water samples. Anal Lett 29:635–643. https://doi.org/10.1080/00032719608000426
Soylak M, Elci L, Dogan M, Mustafa Soylak S (2000) A Sorbent Extraction Procedure for the Preconcentration of Gold, Silver and Palladium on an Activated Carbon Column. Anal Lett 33:513–525. https://doi.org/10.1080/00032710008543070
Soylak M, Elçi L, Doğan M (1996) Determination of some trace metal impurities in refined and unrefined salts after preconcentration onto activated carbon. Fresenius Environ Bull 5:148–155
Soylak M (1998) Soylak, M. "Determination of trace amounts of copper in metallic aluminium samples after separation and preconcentration on an activated carbon column. Fresenius Environ Bull 7:383–387
Hu Y, Zhu Y, Zhang Y, et al (2019) An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour Technol 288:.https://doi.org/10.1016/J.BIORTECH.2019.121511
Balarak D, McKay G (2021) Utilization of MWCNTs_Al2O3 as adsorbent for ciprofloxacin removal_ equilibrium, kinetics and thermo _ Enhanced Reader.pdf. J Environ Sci Heal Part A 56:324–333
Ji H, Wang T, Huang T et al (2021) Adsorptive removal of ciprofloxacin with different dissociated species onto titanate nanotubes. J Clean Prod 278:123924. https://doi.org/10.1016/J.JCLEPRO.2020.123924
Cai Y, Yan Z, Ou Y et al (2022) Effects of different carbon sources on the removal of ciprofloxacin and pollutants by activated sludge: Mechanism and biodegradation. J Environ Sci 111:240–248. https://doi.org/10.1016/j.jes.2021.03.037
Ma Y, Li M, Li P, et al (2021) Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour Technol 319:.https://doi.org/10.1016/J.BIORTECH.2020.124199
Luo K, Pang Y, Yang Q et al (2019) Enhanced ciprofloxacin removal by sludge-derived biochar: Effect of humic acid. Chemosphere 231:495–501. https://doi.org/10.1016/j.chemosphere.2019.05.151
Velusamy K, Periyasamy S, Kumar PS et al (2021) Analysis on the removal of emerging contaminant from aqueous solution using biochar derived from soap nut seeds. Environ Pollut 287:117632. https://doi.org/10.1016/J.ENVPOL.2021.117632
Egbedina AO, Adebowale KO, Olu-Owolabi BI et al (2021) Green synthesis of ZnO coated hybrid biochar for the synchronous removal of ciprofloxacin and tetracycline in wastewater. RSC Adv 11:18483–18492. https://doi.org/10.1039/D1RA01130H
Li J, Yu G, Pan L et al (2018) Study of ciprofloxacin removal by biochar obtained from used tea leaves. J Environ Sci (China) 73:20–30. https://doi.org/10.1016/J.JES.2017.12.024
Šabanović E, Memić M, Sulejmanović J, Huremović J (2016) Sorption of Metals on Pulverized Pumpkin ( Cucurbita pepo L.) Peels. Anal Lett 49:2446–2460. https://doi.org/10.1080/00032719.2016.1152580
Hameed BH, El-Khaiary MI (2008) Removal of basic dye from aqueous medium using a novel agricultural waste material: Pumpkin seed hull. J Hazard Mater 155:601–609. https://doi.org/10.1016/j.jhazmat.2007.11.102
Okoye AI, Ejikeme PM, Onukwuli OD (2010) Lead removal from wastewater using fluted pumpkin seed shell activated carbon: Adsorption modeling and kinetics. Int J Environ Sci Technol 7:793–800. https://doi.org/10.1007/BF03326188
Kowalkowska A, Jóźwiak T (2019) Utilization of pumpkin (Cucurbita pepo) seed husks as a low-cost sorbent for removing anionic and cationic dyes from aqueous solutions. Desalin Water Treat 171:397–407. https://doi.org/10.5004/dwt.2019.24761
Rashid J, Tehreem F, Rehman A, Kumar R (2019) Synthesis using natural functionalization of activated carbon from pumpkin peels for decolourization of aqueous methylene blue. Sci Total Environ 671:369–376. https://doi.org/10.1016/j.scitotenv.2019.03.363
Çelekli A, Bozkuş B, Bozkurt H (2019) Development of a new adsorbent from pumpkin husk by KOH-modification to remove copper ions. Environ Sci Pollut Res 26:11514–11523. https://doi.org/10.1007/s11356-017-1160-2
Bal D, Özer Ç, İmamoğlu M (2021) Green and Ecofriendly Biochar Preparation from Pumpkin Peel and Its Usage as an Adsorbent for Methylene Blue Removal from Aqueous Solutions. Water Air Soil Pollut 232:1–16. https://doi.org/10.1007/s11270-021-05411-w
Ozer C, Imamoglu M, Turhan Y, Boysan F (2012) Removal of methylene blue from aqueous solutions using phosphoric acid activated carbon produced from hazelnut husks. Toxicol Environ Chem 94:1283–1293. https://doi.org/10.1080/02772248.2012.707656
Ashiq A, Sarkar B, Adassooriya N et al (2019) Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 236:124384. https://doi.org/10.1016/J.CHEMOSPHERE.2019.124384
Huang L, Wang M, Shi C et al (2014) Adsorption of tetracycline and ciprofloxacin on activated carbon prepared from lignin with H3PO4 activation. Desalin Water Treat 52:2678–2687. https://doi.org/10.1080/19443994.2013.833873
Kaya N, Uzun ZY (2020) Investigation of effectiveness of pine cone biochar activated with KOH for methyl orange adsorption and CO2 capture. Biomass Convers Biorefinery 1–17.https://doi.org/10.1007/s13399-020-01063-8
Baytar O, Ceyhan AA, Şahin Ö (2020) Production of activated carbon from Elaeagnus angustifolia seeds using H3PO4 activator and methylene blue and malachite green adsorption. Int J Phytoremediation 1–11.https://doi.org/10.1080/15226514.2020.1849015
Sadhu M, Bhattacharya P, Vithanage M, Padmaja Sudhakar P (2021) Adsorptive removal of fluoride using biochar – A potential application in drinking water treatment. Sep Purif Technol 278:119106. https://doi.org/10.1016/j.seppur.2021.119106
Nguyen XC, Nguyen TTH, Nguyen THC et al (2021) Sustainable carbonaceous biochar adsorbents derived from agro-wastes and invasive plants for cation dye adsorption from water. Chemosphere 282:131009. https://doi.org/10.1016/j.chemosphere.2021.131009
Hasana NH, Wahi R, Yusof Y, Mubarak NM (2021) Magnesium-Palm Kernel Shell Biochar Composite for Effective Methylene Blue Removal: Optimization via Response Surface Methodology. Pertanika J Sci Technol 29:1451–1473. https://doi.org/10.47836/pjst.29.3.28
Saeed AAH, Harun NY, Sufian S et al (2021) Production and characterization of rice husk biochar and kenaf biochar for value-added biochar replacement for potential materials adsorption. Ecol Eng Environ Technol 22:1–8. https://doi.org/10.12912/27197050/132099
Sarchami T, Batta N, Rehmann L, Berruti F (2021) Removal of phenolics from aqueous pyrolysis condensate by activated biochar. Can J Chem Eng 99:2368–2385
Quyen V thi, Pham T-H, Kim J, et al (2021) Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere 131312.https://doi.org/10.1016/j.chemosphere.2021.131312
Lua AC, Yang T (2004) Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J Colloid Interface Sci 274:594–601. https://doi.org/10.1016/j.jcis.2003.10.001
Wibawa PJ, Nur M, Asy’ari M, et al (2021) Green synthesized silver nanoparticles immobilized on activated carbon nanoparticles: Antibacterial activity enhancement study and its application on textiles fabrics. Molecules 26
Liu Y, Dong C, Wei H et al (2015) Adsorption of levofloxacin onto an iron-pillared montmorillonite (clay mineral): Kinetics, equilibrium and mechanism. Appl Clay Sci 118:301–307. https://doi.org/10.1016/j.clay.2015.10.010
Afzal MZ, Sun XF, Liu J et al (2018) Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci Total Environ 639:560–569. https://doi.org/10.1016/J.SCITOTENV.2018.05.129
Azargohar R, Dalai AK (2006) Biochar as a precursor of activated carbon. Applied Biochemistry and Biotechnology. Twenty-Seventh Symp Biotechnol Fuels Chem 131:762–773. https://doi.org/10.1385/ABAB:131:1:762
Li R, Wang Z, Guo J et al (2018) Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci Technol 77:1127–1136. https://doi.org/10.2166/WST.2017.636
Peng X, Hu F, Lam FLY et al (2015) Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J Colloid Interface Sci 460:349–360. https://doi.org/10.1016/J.JCIS.2015.08.050
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. K Sven Vetenskapsakademiens Handl 24:1–39
Ho Y, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Weber WJ, Morris JC (1963) Kinetics of adsorption carbon from solutions. J Sanit Eng Div 89:31–60
Tuzen M, Sarı A, Saleh TA (2018) Response surface optimization, kinetic and thermodynamic studies for effective removal of rhodamine B by magnetic AC/CeO2 nanocomposite. J Environ Manage 206:170–177. https://doi.org/10.1016/J.JENVMAN.2017.10.016
Mahmood T, Din SU, Naeem A et al (2014) Kinetics, equilibrium and thermodynamics studies of arsenate adsorption from aqueous solutions onto iron hydroxide. J Ind Eng Chem 20:3234–3242. https://doi.org/10.1016/J.JIEC.2013.12.004
Duran C, Ozdes D, Gundogdu A et al (2011) Tea-industry waste activated carbon, as a novel adsorbent, for separation, preconcentration and speciation of chromium. Anal Chim Acta 688:75–83. https://doi.org/10.1016/j.aca.2010.12.029
Wu J, Wang L, Zhou J et al (2013) Recovery of acetoin from the aqueous solution by means of a novel hyper-cross-linked resin: Equilibrium and kinetics. J Food Eng 119:714–723. https://doi.org/10.1016/J.JFOODENG.2013.07.002
Ozer C, Imamoglu M (2017) Adsorptive transfer of methylene blue from aqueous solutions to hazelnut husk carbon activated with potassium carbonate. Desalin Water Treat 94:236–243. https://doi.org/10.5004/dwt.2017.21582
Masrura SU, Dissanayake P, Sun Y et al (2020) Sustainable use of biochar for resource recovery and pharmaceutical removal from human urine: A critical review. Crit Rev Environ Sci Technol 51:3016–3048. https://doi.org/10.1080/10643389.2020.1818497
Biswas S, Siddiqi H, Meikap BC et al (2020) Preparation and Characterization of Raw and Inorganic Acid-Activated Pine Cone Biochar and Its Application in the Removal of Aqueous-Phase Pb2+ Metal Ions by Adsorption. Water Air Soil Pollut 231:1–17. https://doi.org/10.1007/s11270-019-4375-7
Taşar Ş, Özer A (2020) A Thermodynamic and Kinetic Evaluation of the Adsorption of Pb ( II ) Ions Using Peanut ( Arachis Hypogaea ) Shell-Based Biochar from Aqueous Media. Pol J Environ Stud 29:293–305. https://doi.org/10.15244/pjoes/103027
Langmuir I (1918) The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the Adsorption in Solution. J Phys Chem 57:1100–1107
Saleh TA, Al-Ruwayshid SH, Sarı A, Tuzen M (2020) Synthesis of silica nanoparticles grafted with copolymer of acrylic acrylamide for ultra-removal of methylene blue from aquatic solutions. Eur Polym J 130:109698. https://doi.org/10.1016/J.EURPOLYMJ.2020.109698
Ahmed MB, Zhou JL, Ngo HH, Guo W (2015) Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci Total Environ 532:112–126. https://doi.org/10.1016/J.SCITOTENV.2015.05.130
Arif M, Liu G, Zia urRehman M et al (2022) Carbon dioxide activated biochar-clay mineral composite efficiently removes ciprofloxacin from contaminated water - Reveals an incubation study. J Clean Prod 332:130079. https://doi.org/10.1016/J.JCLEPRO.2021.130079
Hettithanthri O, Rajapaksha AU, Keerthanan S et al (2022) Colloidal biochar for enhanced adsorption of antibiotic ciprofloxacin in aqueous and synthetic hydrolyzed human urine matrices. Chemosphere 297:133984. https://doi.org/10.1016/J.CHEMOSPHERE.2022.133984
Usanmaz S, Özer Ç, İmamoğlu M (2021) Removal of Cu(II), Ni(II) and Co(II) ions from aqueous solutions by hazelnut husks carbon activated with phosphoric acid. Desalin Water Treat 227:300–308. https://doi.org/10.5004/dwt.2021.27303
Yadav S, Asthana A, Singh AK, et al (2021) Adsorption of cationic dyes, drugs and metal from aqueous solutions using a polymer composite of magnetic/β-cyclodextrin/activated charcoal/Na alginate: Isotherm, kinetics and regeneration studies. J Hazard Mater 409:.https://doi.org/10.1016/J.JHAZMAT.2020.124840
Acknowledgements
The authors thank Deva Holding (Istanbul, Turkey) for supplying ciprofloxacin. Ç.Ö. thanks to Sakarya University for providing the opportunity to study.
Author information
Authors and Affiliations
Contributions
Author contribution ÇÖ contributed to experimentation/investigation, data collection and analysis, methodology, conceptualization, writing (original draft preparation), visualization and writing (reviewing and editing); Mİ contributed to supervision, conceptualization, visualization, writing (reviewing and editing) and resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özer, Ç., İmamoğlu, M. Removal of ciprofloxacin from aqueous solutions by pumpkin peel biochar prepared using phosphoric acid. Biomass Conv. Bioref. 14, 6521–6531 (2024). https://doi.org/10.1007/s13399-022-02832-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02832-3