Abstract
Biosynthesis of antioxidant nanoparticles using plant extracts is a simple, rapid, environmentally friendly, and cost-effective approach. In this study, in vitro antioxidant copper mixed oxide nanoparticles (CuO/Cu2O) were prepared from the alcoholic extract of Phoenix Dactylifera L. and different aqueous concentrations of CuSO4·5H2O. The composition, crystallinity, morphology, and particle size of CuO/Cu2O NPs were tuned by increasing the CuSO4·5H2O concentration from 4 to 10 mM. Ultraviolet–visible (UV–Vis) and Fourier-transform infrared (FTIR) spectroscopy confirmed the reduction of CuSO4·5H2O and the formation of the CuO/Cu2O NPs. X-ray diffraction (XRD) confirmed the crystalline nature of the CuO/Cu2O NPs with a crystallite size varying from 18 to 35 nm. Scanning electron micrographs (SEM) showed that the CuO/Cu2O NPs have a spherical morphology with particle sizes ranging from 25 to 100 nm. The best antioxidant CuO/Cu2O NPs have a phase ratio of about 1:1 CuO/Cu2O with a half-maximal inhibitory concentration (IC50) of 0.39 mg/ml, an iron-containing reducing antioxidant power (FRAP) of 432 mg EFeSO4/100 mg NPs, and a total antioxidant capacity (TAC) of 65 mg EAA/gNPs. The results suggest that the synthesized CuO/Cu2O NPs are excellent antioxidants for therapeutic applications.
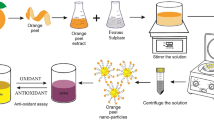
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nature has provided ways and insights into the simple and green synthesis of nanoparticles (NP) [1, 2]. Plant extract and microorganism synthesis has gained extensive attention as a green, sustainable, and eco-friendly protocol for synthesizing a wide range of nanomaterials [3, 4]. To date, many successful attempts have been made to biosynthesize metal and metal oxide NPs using plant extracts (leaves, roots, flowers) and microorganisms (fungi, bacteria, and microalgae) [5, 6]. In contrast to synthesis using microbes, plant extracts are the best candidates for green biosynthesis because the extracts contain biomolecules (polyphenols, terpenoids, phenolic acids, and alkaloids) that oxidize or reduce the metal ions and stabilize the dispersible nanoparticles after the synthesis process [7, 8]. The production cost is lower in plant extract–mediated synthesis because the raw materials (especially plants) are readily available and no additional purification process is required compared to microbial-mediated synthesis [9, 10]. Moreover, this strategy is a sustainable and safe strategy for nanoparticle production because the crystal growth and dispersion of nanoparticles can be easily controlled [11, 12]. In several cases, the synthesis mediated by plant extracts has been shown to improve the biological properties (bioavailability, biocompatibility, cell internalization, antioxidant activity) of the metal and metal oxide nanoparticles and reduce toxicity [13,14,15].
Nowadays, several research groups focus on the green synthesis of metal and metal oxide nanoparticles using plant extract for biomedical applications [16,17,18,19,20,21,22,23,24]. The extracts of medicinal plants have been shown to possess acceptable antioxidant activity, which results from free radical degradation [25]. Metal-based nanoparticles functionalized with plant extracts as natural antioxidants were found to provide targeted delivery and antibacterial activity [26, 27]. Among different antioxidant metal oxide nanoparticles, CuO and Cu2O NPs have attracted significant attention due to their low cost, abundant availability of copper salt, and high antioxidant activity of CuO and Cu2O NPs [28]. Antioxidant properties of CuO and Cu2O NPs depend on their nature, polymorphism, crystal structure, chemical composition, surface charge, particle size, surface-to-volume ratio, surface coating, and dispersion state [29]. Aside from antioxidant applications, CuO and Cu2O NPs were reported for the treatment of cancer diseases [30], oxidative stress diseases [31], and cardiovascular diseases [15, 32]. CuO and Cu2O NPs also offer many other applications, including photocatalysis, antibacterial, biosensing, and energy storage [33, 34].
To date, several physical and chemical processes are currently used for CuO and Cu2O NP synthesis, which allows for obtaining particles with preferred properties [35]. However, these production methods have many disadvantages due to hazardous organic solvents, a costly reagent, the difficulty of isolating the nanoparticles, and the longer time required [36]. Herefore, there is an essential need for a clean, reliable, cost-effective, and environmentally friendly process for the synthesis of nanoparticles [37]. The plant extract–mediated synthesis of CuO and Cu2O NPs using plant extracts such as Anthemis nobilis [38], Calotropis gigantea [39], Gloriosa superba [40], Aloe vera [37], Carica papaya [41], and Emblica officinalis [42]. The microbe-mediated synthesis of CuO and Cu2O NPs has also been reported. However, the major drawback of microbe-mediated synthesis is the complex procedures, i.e., microbial growth conditions, and isolation of microorganisms, which require a longer reaction time and thus increase the cost of scaling up [43].
Previous research has shown that both CuO and Cu2O NPs have strong antioxidant activity, but the antioxidant properties of copper mixed oxides (CuO/Cu2O NPs) have not yet been investigated. This work aims to synthesize CuO/Cu2O NPs by an environmentally friendly method using a leaf extract of Phoenix dactylifera L. and investigate the effects of extract/precursor contractions on the size, shape, and crystal structure, and estimate their antioxidant activity. The phytochemical constituents of Phoenix dactylifera L. act as bio-reducing agents and stabilizers for the formation of CuO/Cu2O NPs, which showed good antioxidant and cytotoxic activities. The synthesized CuO/Cu2O NPs were characterized to determine the properties of bioactive constituents (capping agents) in each leaf extract [44]. The method presented here is an alternative to the solvothermal synthesis of CuO and Cu2O NPs in the context of green chemistry. Phoenix dactylifera L is one of the most important plant sources rich in active compounds such as polyphenols, terpenoids, phenolic acids, and alkaloids, all of which have significant antioxidant activity in biological systems [45]. Copper is one of the useful and essential substances for the human body, providing balance and stability in tissues. CuO and Cu2O NPs have been introduced by the US Environmental Protection Agency (EPA) as effective antibacterial agents [45]. Accordingly, the synthesized CuO/Cu2O NPs can be used as drug carriers (chemotherapeutics) for antioxidant and antimicrobial applications.
2 Experimental
2.1 Chemicals, reagents, and plant materials
Phoenix dactylifera L. leaves of Ghars quality were collected from El Oued, Southeast of Algeria (latitude: 31°–34° N, longitude: 6°–8° E). Copper sulfate (CuSO4·5H2O, 98%, Sigma-Aldrich), ethanol absolute (C2H5OH, 99%, Sigma-Aldrich), methanol (CH3OH, 96%, Sigma-Aldrich), sodium phosphate monobasic (NaH2PO4, 99%, Biochem Chemopharma), sulfuric acid (H2SO4, 96%, Biochem Chemopharma), hydrochloric acid (HCl, 35%, Biochem Chemopharma), ascorbic acid (C6H8O6, 99%, Biochem Chemopharma), acetic acid (CH3COOH, 99.5%, Biochem Chemopharma), sodium acetate (CH3COONa, 99%, Biochem Chemopharma), ammonium molybdate ((NH4)6Mo7O24, 99%, Sigma-Aldrich), 2,2-diphenyl-1-picrylhydrazyl (DPPH, C18H12N5O6, 95%, Alfa Aesar), iron(III) chloride (FeCl3, 99%, Biochem Chemopharma), iron(II) sulfate (FeSO4·7H2O, 99%, Biochem Chemopharma), and 2,4,6-tripyridyl-s-triazine (TPTZ, C18H12N6, 98%, Alfa Aesar).
2.2 Preparation of plant extract
The leaves of Phoenix Dactylifera L. were rinsed several times with distilled water and then dried at room temperature and protected from sunlight for 5 days to preserve the chemical composition as much as possible [46]. Briefly, 10 g of powder of these leaves was added to 60 ml of ethanol/water mixture (70%/30%). This preparation was shaken at room temperature for 24 h. The extracts were then filtered using Whatman No. 1 filter paper and stored at 4 °C.
3 Biosynthesis of copper mixed oxide nanoparticles
For the synthesis of CuO/Cu2O NPs, four different samples were prepared with different ratios of CuSO4·5H2O solution to Phoenix Dactylifera L. (v/v). Specifically, 1 ml of the extract of Phoenix dactylifera L. was added to 30 ml of aqueous CuSO4·5H2O solution (4, 6, 8, and 10 mM). For each sample, the mixture was stirred for 2 h at 70 °C with a magnetic stirrer until the color turned to deep brown and a precipitate was observed. The precipitate of each sample was centrifuged at 1000 rpm for 30 min and washed with deionized water to remove impurities and centrifuged again for 20 min. The brown precipitate obtained was dried at 80 °C for one night and then annealed in air at 400 °C for 2 h [46].
4 Characterization of copper mixed oxide nanoparticles
The crystalline structure of CuO/Cu2O NPs was examined by using X-ray diffraction (XRD, Rigaku Miniflex 600) using CuKα radiation (40 kV and 30 mA) with a wavelength of 1.5418 A and scanning speed of 0.5° [47]. The particle size and shape were analyzed using a scanning electron microscope (SEM, TESCAN VEGA 3). The bonding characteristic of CuO/Cu2O NPs was analyzed using a Fourier transform infrared spectrometer (FTIR, Nicolet iS5, Thermo Fisher Scientific) in a spectral range of 4000–500 cm−1. The light absorbance and bandgap energy of CuO/Cu2O NPs were determined by the UV–vis absorption spectrum (Shimadzu UV-1800s) in the wavelength range of 200–800 nm.
5 Total antioxidant capacity
The total antioxidant capability (TAC) of the samples was estimated by the phosphomolybdenum method [35]. The TAC assay is based on the reduction of molybdate ions MoO42− (Mo6+) into green MoO2+ (Mo5+) in the presence of antioxidants (CuO/Cu2O NPs) in an acid milieu [36]. TAC assay usually detects antioxidants such as some phenolics, ascorbic acid, α-tocopherol, and carotenoids. An amount of 0.2 ml of each concentration of CuO/Cu2O NPs has been mixed with 2 ml of reagent solution consisting of 0.6 M H2SO4, 4 mM ammonium molybdate, and 28 mM NaH2PO4. These were incubated at a temperature of 95 °C for 1.5 h; after cooling, it was measured for absorbance at 695 nm. The total antioxidant capacity of the samples was obtained from an ascorbic acid calibration curve. The latter was traced using various ascorbic acid concentrations from 0.01 to 0.1 mg/ml. The total antioxidant capability is represented as milligrams of ascorbic acid equivalence per gram of nanoparticles (mg EAA/g NPs).
6 Ferric reducing antioxidant power
The reducing power of ferric ion was determined by the ferric reducing antioxidant power (FRAP) method prescribed by Benzie and Strain [48]. This method is based on the reduction of Fe3+ ions into Fe2+ ions through the antioxidant (CuO/Cu2O NPs); the reaction is detected by the transfer from the yellow color of Fe3+ ions to the blue Fe2+ ions [48]. The increase in UV–Vis absorbance indicates the elevation of the reducing power of the tested samples. The FRAP solution was prepared by admixing 2.5 ml of 10 mM TPTZ prepared in 40 mM HCl, 2.5 ml of 20 mM FeCl3, 25 ml of acetate buffer (pH ~ 3.6), and 3 ml of distilled water [49]. About 30 µl of the sample (CuO/Cu2O NPs) was mixed with 970 µl of FRAP solution and incubated for 30 min at 37 °C. The reaction was monitored using UV–vis spectroscopy by measuring the absorbance at 593 nm. In this test, iron sulfate (FeSO4) was used as the standard. The results of the reductive power of the samples are presented in milligrams of FeSO4 equivalent per 100 mg of CuO/Cu2O NPs (mg E FeSO4/100 mg NPs) [48].
7 Free radical-scavenging activity (DPPH)
The DPPH assay is a rapid and the most widely used assay for characterizing the antiradical activity of plant extracts [50]. It is expressed as IC50 which denotes the concentration of each sample required to scavenge 50% of DPPH free radicals. The DPPH assay is based on measuring the capacity of antioxidants (CuO/Cu2O NPs) to scavenge the DPPH radical [40]. Briefly, 2 ml of a methanolic solution of 0.1 mM DPPH was mixed with 1 ml with different CuO/Cu2O NP concentrations. The obtained mixture was incubated in the dark for 15 min at room temperature [51]. The absorbance is measured at 517 nm against a control consisting of 1 ml methanol and 2 ml DPPH solution.
The percentage of inhibition is calculated using the following equation:
where.
Abscontrol is the absorbance of the control (containing no antioxidants).
Abssample is the absorbance of the sample after 15 min.
The antiradical activity is then expressed by the IC50 value, where IC50 is the sample concentration necessary to obtain 50% of the reduced form of the DPPH radical.
8 Results and discussion
The Phoenix dactylifera L.–mediated synthesis of CuO/Cu2O NPs is more advantageous than chemical and physical synthesis as it is a clean, non-toxic, cost-effective, and environmentally friendly approach. Moreover, Phoenix dactylifera L. is easily available in nature, and this makes it a preferable plant material for scaling up at the industrial level [20, 24]. Biosynthesis of CuO/Cu2O NPs depends on secondary metabolites (polyphenols, terpenoids, phenolic acids, and alkaloids) contained in the extract, which are also responsible for reducing the metal ions [4]. During synthesis, plant extracts are used as bio-reducing agents and capping agents. The reduction process consists of returning Cu2+ ions to Cu0. The Cu0 represented by the Cu NPs is converted into CuO/Cu2O NPs after annealing in the incinerator at 400 °C for 2 h [52].
The phytochemical analysis of the extract from the leaves of Phoenix dactylifera L. revealed that they contain flavonoids, condensed tannins, and saponins [21]. The most important visual observation during the reaction is the change of the color of the solution from green to brown within 30 min. The brown color is a clear indication of the formation of CuO/Cu2O NPs. Based on this evidence, a possible mechanism for the reduction of Cu2+ and the formation of CuO/Cu2O NPs was proposed using Phoenix dactylifera L. extract (Scheme 1). The antioxidant activity of CuO/Cu2O NPs reflects their ability to scavenge free radicals in the organism. In vitro methods were used to investigate the antioxidant potential of CuO/Cu2O NPs. The dispersion of the nanoparticles was injected into a free radical producing system, and the inhibitory effect on free radicals was measured by three assays, i.e., reducing antioxidant power of iron (FRAP), total antioxidant capacity (TAC), and radical scavenging activity (RSA), for DPPH.
9 Crystal structure and composition
XRD results show variation in crystallite size and composition (phase CuO/Cu2O ratio) related to the change in the concentrations of CuSO4·5H2O. Figure 1 shows the XRD pattern of the CuO/Cu2O NPs prepared using the Phoenix dactylifera L. extract and different concentrations of CuSO4·5H2O. This diffractogram affirms the existence of two crystalline phases, cuprous oxides (Cu2O) and cupric oxide (CuO). The peaks position with 2θ values of 32.5°, 35.5°, 38.8°, 48.8°, 58.3°, 61.7°, 66.3°, and 68.4°, corresponding to the crystalline planes of (110), (002), (111), (202), (202), (113), (311), and (220) which confirm the formation of the monoclinic crystal structure for CuO (JCPDS-01–089-5899) [53]. The other five characteristic peaks at 2θ values of 29.5°, 36.3°, 42.4°, 61.3°, and 73.4° are attributed to the crystal planes of (110), (111), (200), (220), and (311), which correspond to the cubic phase Cu2O (JCPDS-00–005-0667). The crystallite size was calculated from the full width at half maximum intensity (FWHM) measured on the corrected diffraction profile using the D = Kλ/(β cos θ), using the Scherrer formula [54], as shown in Table 1. For the cubic crystal structure, K = 0.94, λ wavelength of X-ray; d = the full width at FWHM of the peak [55].
XRD results show that the crystallite sizes increase for the CuO (from 15.7 to 23.0) and Cu2O (from 18.3 to 35.4 nm) phases which slightly increase as the CuSO4·5H2O concentration increases from 4 to 10 mM. Interestingly, the ratio of the CuO/Cu2O phase increases from 58/42 to 98/2 with increasing the CuSO4·5H2O concentration from 4 to 10 mM (Table 1). Typically, increasing the CuSO4·5H2O concentration (reactant concentration) accelerates the nucleation rate and shortens the reaction time; thus, nanocrystals with larger crystallite sizes are formed. Similarly, increasing the CuSO4·5H2O concentration (reactant concentration) provides more opportunity to produce the CuO phase rather than the Cu2O phase. This can explain the increase in the CuO/Cu2O phase (from 58/42 to 98/2) by increasing the CuSO4·5H2O concentration (from 4 to 10 mM). Table 1 shows the average crystallite size, crystal shape, and phase ratio CuO/Cu2O of the prepared CuO/Cu2O NPs.
FTIR analysis was carried out to confirm the formation of CuO/Cu2O NPs using the Phoenix dactylifera L. extract as a reducing and stabilizing agent. Figure 2 brings together the FT-IR spectrum of Phoenix dactylifera L. leaf extract with different spectra of CuO/Cu2O NPs prepared at different ratios before thermal treatment at 400 °C [56]. FTIR spectra (Fig. 2) of the Phoenix dactylifera L. extract exhibited several absorption bands (at 3264, 1605, 1442, 1283, 1049, and 671 cm−1) corresponding to the functional groups of the biomolecules existing in the plant extract. The broad and strong band at 3264 cm−1 is attributed to hydrogen-bonded OH groups of alcohols and phenols as well as the presence of amide N–H amines [56]. The bands at 1605 and 1442 cm−1 are attributed to the (C = O) stretching of the amide carbonyl and the C–N stretching vibration of the aromatic amine [41]. A weak band at 1283 and 1049 cm−1 is attributed to C–O stretching and C–O–C stretching asymmetric vibration, respectively [57]. The 671-cm−1 band corresponds to the aromatic (C–H) group [58].
The results of FTIR analysis of the prepared CuO/Cu2O NPs at the different concentrations are shown in Fig. 2. The bands situated at 512 cm−1 and 618 cm−1 correspond to CuO vibrations, which confirm the formation of CuO/Cu2O NPs [59], which is in good agreement with literature values. Previous results showed that three characteristic peaks of the vibrations of Cu–O were observed at 421 cm−1, 472 cm−1, and 618 cm−1. However, the FTIR spectrum of Cu2O NPs shows only one peak at about 533.6 cm−1 attributed to Cu–O vibration [58]. The other absorption band at 1200 cm−1 can confirm the existence of the carboxylic acid group [58]. The peak absorption at 1730 cm−1 may be due to C = C stretching vibrations around the C = O amide–conjugated C = O of the proteins involved in the reduction and stabilization process [41]. The peak at 1596 cm−1 represented the C = O stretching of the ketone group. FTIR spectra show that the synthesized CuO/Cu2O NPs might be stabilized through the interactions of –OH and C = O groups in the carbohydrates, flavonoids, tannins, and phenolic acids present in Phoenix dactylifera L.
10 Morphology and particle size
The morphology and particle size of the CuO/Cu2O NPs may be affected by several factors, CuO/Cu2O NPs including pH of the solution, temperature, concentration of the Phoenix dactylifera L. extract used, and concentrations of CuSO4·5H2O used [60]. In this work, all these parameters were kept constant and only the CuSO4·5H2O used was varied. The SEM images (Fig. 3) indicated that they were used to study the formation of CuO/Cu2O NPs and their morphological size. Figure 3 shows the SEM images showing concentrations of CuSO4·5H2O used to have a significant effect on the particle size, and size distribution of the CuO/Cu2O NPs produced. Table 1 shows the particle size and particle shape of the prepared CuO/Cu2O NPs after annealing.
As shown in Fig. 3, the average particle size of the CuO/Cu2O NPs gradually increases (from 25 to 100 nm) when CuSO4·5H2O is increased from 4 to 10 mM. The particle morphology of CuO/Cu2O NPs was slightly changed by increasing the CuSO4·5H2O concentration from 4 to 10 mM. At concentrations of 4, 6, and 8 mM (Fig. 3a–c), the morphology of CuO/Cu2O NPs is mainly spherical with different sizes and less agglomeration. On the other hand, at a concentration of 10 mM (Fig. 3d), spherical and rhombohedral shapes with particle size distribution abroad (80–150 nm) were observed. The sample prepared with 4 mM CuSO4·5H2O shows a narrow particle size distribution, which broadens with the increase in CuSO4·5H2O concentration from 4 to 10 mM. As shown in Table 1, the particle size of the CuO/Cu2O NPs with a concentration of 4 mM closely matches the crystallite size calculated from the XRD pattern. This indicates that the 4-mM samples are monocrystalline. The large difference between the crystallite size and the particle size of the 10-mM sample indicates that the CuO/Cu2O NPs prepared at this concentration are polycrystalline.
Flavonoids, condensed tannins, and saponins in the Phoenix dactylifera L. extract are well bound with CuO/Cu2O NPs. These compounds are promising candidates for the reducing and stabilizing of CuO/Cu2O NPs. Stabilization of the CuO/Cu2O NPs is probably due to the binding of the flavonoids, condensed tannins, and saponin molecules to the surface of the CuO/Cu2O NPs. Previous studies reported similar results for the biosynthesis of CuO and Cu2O NPs from other plant extracts. However, these studies did not include the antioxidant activity in the synthesis of CuO and Cu2O. Chinnaiah et al. [61] biosynthesized Cu2O NPs rather than CuO using Datura metel L. and found that the average crystallite size of Cu2O NPs is about 19.56 nm. Kumar and coworkers [62] used Andean sacha inchi (Plukenetia volubilis L.) leaves to prepare monodispersed semicrystalline Cu2O NPs under heating. However, the observed crystallite size of the Cu2O NPs is ~ 46 nm. Ananda Murthy et al. [63] synthesized CuO NPs with a monoclinic structure and a particle size of 19.7 nm using Vernonia amygdalina Del. extract. However, the synthesis of mixed Cu2O/CuO NPs has not been extensively investigated yet. Xolile Fuku [64] biosynthesized Cu/Cu2O/CuO NPs using pomegranate peel extract. The average crystallite size of Cu/Cu2O/CuO NPs was about 20–25 nm which is comparable with our results.
11 UV–visible absorbance and bandgap energy
Cupric oxide (CuO) is a transition metal oxide with a monoclinic structure and a narrow bandgap of 1.3 to 1.7 eV, while the Cu2O NPs have a direct bandgap of 2.0 to 2.5 eV. Generally, several factors may affect the bandgaps of the prepared Cu2O NPs and CuO NPs, i.e., crystallinity, crystallite size, particle size, particle shape, and composition [65]. The small bandgap energies allow Cu2O and CuO NPs to absorb the vast majority of the solar spectrum, and the direct bandgaps endow the CuO/Cu2O NPs with a large absorption coefficient. Figure 4 shows the UV–Vis spectrum of CuO/Cu2O NPs using the Phoenix dactylifera L. leaf extracts at different CuSO4·5H2O concentrations. The UV–vis spectra of all the samples show a strong absorption peak at 275 nm, attributed to the surface plasmon resonance of CuO/Cu2O NPs. This latter is caused by the collective oscillation of the electrons in the free conduction band which is excited by the incident electromagnetic radiation. Also, as observed, the absorption intensity of the samples increased with an increase in copper concentration. This suggests an increase in the number of nanoparticles formed as a result of the reduction of copper ions [40, 62]. The bandgap energy of the prepared samples was determined according to relation (4) [66]:
where α is the absorption coefficient, \(h\) is the Planck’s constant, \(v\) is the frequency of vibration, A is the proportional constant, \({E}_{g}^{\mathrm{opt}}\) is the optical bandgap energy, and n is a constant that denotes the nature of the electron transition, i.e., n = 2 for the direct transmission, and n = 1/2 for the indirect transmission as shown in Fig. 4b, c. By plotting \({(\alpha h\nu )}^{2}\) and \({(\alpha h\nu )}^{1/2}\) versus photon energy (\(hv\)), the optical energy bandgap for the direct \({E}_{g1}^{\mathrm{opt}}\) and indirect \({E}_{g2}^{\mathrm{opt}}\) transition can be determined, respectively [67]. The value of \({E}_{g}^{\mathrm{opt}}\) are obtained by extrapolating to \({(h\nu \alpha )}^{2}=0\) for direct transition and \({(\alpha h\nu )}^{1/2}=0\) for the indirect transition as shown in Fig. 4b, c, respectively, and also shown in Table 3. As the CuSO4·5H2O concentration increases from 4 to 10 mM, the direct bandgap increases from 1.92 to 2.38 eV, and the indirect bandgap also increases from 1.74 to 1.87 eV. Both direct and indirect energy gap values for the as-synthesized CuO/Cu2O NP samples exhibit a bandgap of 1.3–2.5 eV, as per previous studies [64]. The results are consistent with the literature review that the bandgap increases with a decrease in particle size [68].
Urbach energy is sometimes known as Urbach’s tail and can be detected by UV–vis spectra. The higher value of Urbach energy shows lower crystallinity and disorder in the CuO/Cu2O NPs. The Urbach energy \({E}_{u}\) is determined by taking the reciprocal values of the slopes of the linear part of the \(\mathrm{ln}(a)\) versus photon energy (Fig. 4d) [46]. The estimated Urbach energy values for the samples are shown in Table 2.
The plot of \(\mathrm{ln}(a)\) versus hν of the CuO/Cu2O NPs samples is shown in Fig. 4d. The Urbach energy \({E}_{u}\) was calculated by reciprocating the slope of the linear portion in the photon energy of the curve. The latter is determined as the difference in energy between the ends of the tails of the valence and conduction bands: with the decrease in this energy, being disordered, the disorder can also change depending on the addition of modifying oxides. Table 2 shows that the Urbach energy of the CuO/Cu2O NPs slightly decreases from 0.533 to 0.369 eV with the increase in the particle size (from 25 to 100 nm) and the increase in CuSO4·5H2O concentrations from 4 to 10 mM. These results were explained for Urbach energy due to the effect of structural and thermal perturbation.
12 Evaluation of the antioxidant activity
Table 3 shows the TAC, FRAP, and IC50 DPPH (mg/ml) results of the prepared CuO/Cu2O NPs. The TAC measurements showed that all the synthesized CuO/Cu2O NPs exhibit a significant antioxidant capacity, and the best sample was synthesized at a concentration of 6 mM CuSO4·5H2O with a value of 65.1 ± 3.1 mg EAA/g NPs (see Fig. 5). Cu deficiency affects the antioxidant function of the body and leads to a variety of diseases [69]. The prepared CuO/Cu2O NPs can increase the Cu content in the blood of Cu-deficient patients, and provide improved antioxidant activity. The CuO/Cu2O NPs affect on the cell membrane composition in a pathway to further protect the integrity of the cell membrane structure and function, as well as the tissues and organs. Min and coworkers [69] studied the effects of copper oxide (Cu2O NPs) on the antioxidant function of Cu-deficient Kazakh sheep, where the Cu content in the blood, wool, and liver of Cu-deficient Kazakh sheep was significantly lower than that of healthy animals. The authors supplemented the Kazakh sheep with Cu2O NPs or CuSO4, and as result, the blood Cu concentration increased significantly. Interestingly, from the 5th day, the Cu content of the Cu2O groups was significantly higher than that of the CuSO4 group [69].
Figure 5 b shows the FRAP results of the prepared CuO/Cu2O NPs. The FRAP assay measures the antioxidant activity by reducing Fe3+ ions to Fe2+ ions by the CuO/Cu2O NPs. Following the reduction of the ferric iron, a blue color develops that can be monitored colorimetrically at 594 nm. The reducing power (FRAP) for different samples varied from 354 to 432 E FeSO4/100 mg NPs, while the concentration at 6 mM gave the most important reducing activity (432 mg E FeSO4/100 mg NPs) (see Fig. 5). In a similar study, Ijaz and coworkers [70] synthesized CuO NPs using Abutilon indicum leaf extract. The authors found that the maximum antioxidant activity value of 9.10 TE/ml was observed by 1000 µg CuO NPs while the minimum value (0.65 ± 0.01 TE/ml) was obtained for 60 µg CuO NPs [70].
Figure 5 c shows the antioxidant activity of the CuO/Cu2O NPs using a DPPH assay. The DPPH assay is used to predict antioxidant activities via a mechanism in which antioxidants inhibit lipid oxidation. The lower IC50 value indicates a stronger ability of CuO/Cu2O NPs to act as DPPH scavengers, whereas the higher IC50 value indicates a lower scavenging activity of CuO/Cu2O NPs. The effect of the different CuO/Cu2O NPs on the antioxidant activity of DPPH radicals is shown in Table 2. As shown in Fig. 5d, the DPPH activity of the CuO/Cu2O NPs was found to increase in a dose-dependent manner. Based on the IC50 results, the CuO/Cu2O NPs synthesized from 6 mM CuSO4·5H2O showed the lowest IC50 value in the order of (0.386 mg/ml), which is evidence that this concentration exhibits anti-free radical activity.
By comparing these results with those obtained in the TAC, FRAP, and IC50 DPPH assays, we can conclude that the CuO/Cu2O NPs prepared from 6 mM CuSO4·5H2O provides a strong antioxidant activity, which confirms that grain size has a significant effect on antioxidant potential. In similar studies [70,71,72], researchers evaluated the antioxidant activity of CuO NPs, and by comparing the results obtained, we can say that we got wonderful results. Atoussi and coworkers [73] synthesized CuO NPs by aqueous leaf extract of Portulaca oleracea (L). The authors found that the CuO NPs possessed the reducing capacity when the IC50 value was 68.3 μg/ml and 79.8 μg/ml, respectively, and the anti-inflammatory ability while the IC50 value was 77.5 μg/ml and 60.7 μg/ml, respectively [73].
13 Conclusion
Plant extract–mediated synthesis is a green, simple, and low-cost technique for producing antioxidant nanoparticles. In this work, antioxidant copper mixed oxide nanoparticles (CuO/Cu2O NPs) were prepared from alcoholic extracts of Phoenix dactylifera L. (source of phenolic compounds) and different aqueous concentrations of CuSO4·5H2O. The composition, crystallinity, morphology, and particle size of CuO/Cu2O NPs were adjusted by changing the CuSO4·5H2O concentration (from 4 to 10 mM). The XRD analysis confirmed the existence of two copper oxide phases, monoclinic (CuO) and cubic (Cu2O). The best antioxidant CuO/Cu2O NPs have a particle size of 55 nm and a CuO/Cu2O phase ratio of about 1:1. The antioxidant activity results indicate that the CuO/Cu2O NPs synthesized with the extract of Phoenix dactylifera L. are potent antioxidants and can protect humans against various oxidative stresses.
Abbreviations
- CuO:
-
Cupric oxide
- Cu2O:
-
Cuprous oxide
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FRAP:
-
Ferric reducing antioxidant power
- IC50 :
-
Half-maximal inhibitory concentration
- NPs:
-
Nanoparticles
- TAC:
-
Total antioxidant capacity
References
Rasouli R, Barhoum A, Uludag H (2018) A review of nanostructured surfaces and materials for dental implants: surface coating, patterning and functionalization for improved performance. Biomater Sci 6:1312–1338. https://doi.org/10.1039/c8bm00021b
Meftahi A, Samyn P, Geravand SA et al (2022) Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: formulations, regulations, and emerging applications. Carbohydr Polym 278:118956. https://doi.org/10.1016/J.CARBPOL.2021.118956
Oves M, Ahmar Rauf M, Aslam M et al (2022) Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J Biol Sci 29:460–471. https://doi.org/10.1016/J.SJBS.2021.09.007
Oves M, Aslam M, Rauf MA et al (2018) Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C 89:429–443. https://doi.org/10.1016/j.msec.2018.03.035
Albukhari SM, Ismail M, Akhtar K, Danish EY (2019) Catalytic reduction of nitrophenols and dyes using silver nanoparticles @ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids Surfaces A Physicochem Eng Asp 577:548–561. https://doi.org/10.1016/J.COLSURFA.2019.05.058
Salama A, Abouzeid R, Leong WS et al (2021) Nanocellulose-based materials for water treatment: adsorption, photocatalytic degradation, disinfection, antifouling, and nanofiltration. Nanomater 11:3008. https://doi.org/10.3390/NANO11113008
Jeevanandam J, Chan YS, Danquah MK (2016) Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev 3:55–67. https://doi.org/10.1002/CBEN.201500018
Hamimed S, Abdeljelil N, Landoulsi A, et al (2022) Bacterial cellulose nanofibers. Handb Nanocelluloses 1–38.https://doi.org/10.1007/978-3-030-62976-2_15-1
Jeevanandam J, Kiew SF, Ansah SB et al (2022) Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale. https://doi.org/10.1039/D1NR08144F
Harish V, Tewari D, Gaur M et al (2022) Review on nanoparticles and nanostructured materials: bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomater 12:457. https://doi.org/10.3390/NANO12030457
Rastogi A, Singh P, Haraz FA, Barhoum A (2018) Chapter 19 - Biological synthesis of nanoparticles: an environmentally benign approach. In: Barhoum A, Hamdy Makhlouf ASBT-F of N (eds) Micro and Nano Technologies. Elsevier, pp 571–604. https://doi.org/10.1016/B978-0-323-51255-8.00023-9
Barhoum A, Rehan M, Rahier H et al (2016) Seed-mediated hot-injection synthesis of tiny Ag nanocrystals on nanoscale solid supports and reaction mechanism. ACS Appl Mater Interfaces 8:10551–10561. https://doi.org/10.1021/acsami.5b10405
Tan K, Barhoum A, Pan S, Danquah M (2018) Risks and toxicity of nanoparticles and nanostructured materials. In: Emerging Applications of Nanoparticles and Architecture Nanostructures. pp 121–139. https://doi.org/10.1016/B978-0-323-51254-1.00005-1
Barhoum A, Jeevanandam Jaison, Rastogi A et al (2020) Plant celluloses, hemicelluloses, lignins, and volatile oils for the synthesis of nanoparticles and nanostructured materials. Nanoscale. https://doi.org/10.1039/d0nr04795c
Jeevanandam J, Ling JKU, Barhoum A, et al (2022) Bionanomaterials: definitions, sources, types, properties, toxicity, and regulations. Fundam Bionanomaterials 1–29.https://doi.org/10.1016/B978-0-12-824147-9.00001-7
Bouafia A, Laouini SE (2020) Green synthesis of iron oxide nanoparticles by aqueous leaves extract of Mentha Pulegium L.: effect of ferric chloride concentration on the type of product. Mater Lett 265:127364. https://doi.org/10.1016/J.MATLET.2020.127364
Laouini SE, Bouafia A, Soldatov AV et al (2021) Green synthesized of Ag/Ag2O nanoparticles using aqueous leaves extracts of Phoenix dactylifera L. and their azo dye photodegradation. Membr 468(11):468. https://doi.org/10.3390/MEMBRANES11070468
Bouafia A, Laouini SE, Khelef A et al (2021) Effect of ferric chloride concentration on the type of magnetite (Fe3O4) nanoparticles biosynthesized by aqueous leaves extract of Artemisia and assessment of their antioxidant activities. J Clust Sci 32:1033–1041. https://doi.org/10.1007/S10876-020-01868-7/TABLES/2
Abdullah JAA, Salah Eddine L, Abderrhmane B et al (2020) Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain Chem Pharm 17:100280. https://doi.org/10.1016/J.SCP.2020.100280
Belaiche Y, Khelef A, Laouini SE, et al (2021) Green synthesis and characterization of silver/silver oxide nanoparticles using aqueous leaves extract of Artemisia herba-alba as reducing and capping agents. Rev Română Mater/Rom J Mater 342–352
Bouafia A, Laouini SE, Ouahrani MR (2020) A review on green synthesis of CuO nanoparticles using plant extract and evaluation of antimicrobial activity. Asian J Res Chem 13:65. https://doi.org/10.5958/0974-4150.2020.00014.0
Laid TM, Abdelhamid K, Eddine LS, Abderrhmane B (2021) Optimizing the biosynthesis parameters of iron oxide nanoparticles using central composite design. J Mol Struct 1229:129497. https://doi.org/10.1016/J.MOLSTRUC.2020.129497
Bouafia A, Laouini SE (2020) Plant-mediated synthesis of iron oxide nanoparticles and evaluation of the antimicrobial activity: a review. Mini Rev Org Chem 17:1–11. https://doi.org/10.2174/1570193X17999200908091139
Bouafia A, Laouini SE, Tedjani ML, et al (2021) Green biosynthesis and physicochemical characterization of Fe 3 O 4 nanoparticles using Punica granatum L. fruit peel extract for optoelectronic applications. Text Res J 004051752110066. https://doi.org/10.1177/00405175211006671
Aljabali AAA, Obeid MA, Awadeen SA, et al (2022) Nature bioinspired and engineered nanomaterials. Fundam Bionanomaterials 31–58.https://doi.org/10.1016/B978-0-12-824147-9.00002-9
Bhagat M, Anand R, Datt R et al (2019) Green synthesis of silver nanoparticles using aqueous extract of Rosa brunonii Lindl and their morphological, biological and photocatalytic characterizations. J Inorg Organomet Polym Mater 29:1039–1047. https://doi.org/10.1007/s10904-018-0994-5
Eshgh NA, Meftahi A, Khajavi R, Aljabali AAA, Barhoum A. (2021) Nanocelluloses for tissue engineering and biomedical scaffolds. In: Barhoum A (ed) Handbook of Nanocelluloses. Springer, Cham. https://doi.org/10.1007/978-3-030-62976-2_43-1
Vanathi P, Rajiv P, Sivaraj R (2016) Synthesis and characterization of Eichhornia-mediated copper oxide nanoparticles and assessing their antifungal activity against plant pathogens. Bull Mater Sci 39:1165–1170. https://doi.org/10.1007/S12034-016-1276-X/FIGURES/6
Zhang X, Wang K, Liu M et al (2015) Polymeric AIE-based nanoprobes for biomedical applications: recent advances and perspectives. Nanoscale 7:11486–11508. https://doi.org/10.1039/C5NR01444A
Vaid P, Raizada P, Saini AK, Saini RV (2020) Biogenic silver, gold and copper nanoparticles - a sustainable green chemistry approach for cancer therapy. Sustain Chem Pharm 16:100247. https://doi.org/10.1016/J.SCP.2020.100247
Noman M, Shahid M, Ahmed T, et al (2020) Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol Environ Saf 192.https://doi.org/10.1016/J.ECOENV.2020.110303
Hassan MS, Amna T, Yang OB et al (2012) Smart copper oxide nanocrystals: synthesis, characterization, electrochemical and potent antibacterial activity. Colloids Surf B Biointerfaces 97:201–206. https://doi.org/10.1016/J.COLSURFB.2012.04.032
Karatutlu A, Barhoum A, Sapelkin A (2018) Theories of nanoparticle and nanostructure formation in liquid phase. In: Emerging applications of nanoparticles and architectural nanostructures: current prospects and future trends. Elsevier Inc., pp 597–619. https://doi.org/10.1016/B978-0-323-51254-1.00020-8
Gaur M, Misra C, Yadav AB et al (2021) Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Mater 14:5978. https://doi.org/10.3390/MA14205978
Karatutlu A, Barhoum A, Sapelkin A (2018) Liquid-phase synthesis of nanoparticles and nanostructured materials. In: Emerging applications of nanoparticles and architectural nanostructures: current prospects and future trends. Elsevier Inc., pp 1–28. https://doi.org/10.1016/B978-0-323-51254-1.00001-4
Ghidan AY, Al-Antary TM, Awwad AM (2016) Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: effect on green peach Aphid. Environ Nanotechnology, Monit Manag 6:95–98. https://doi.org/10.1016/J.ENMM.2016.08.002
Kumar PPNV, Shameem U, Kollu P et al (2015) Green synthesis of copper oxide nanoparticles using Aloe vera leaf extract and its antibacterial activity against fish bacterial pathogens. Bionanoscience 5:135–139. https://doi.org/10.1007/S12668-015-0171-Z/FIGURES/5
Nasrollahzadeh M, Maham M, Mohammad Sajadi S (2015) Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J Colloid Interface Sci 455:245–253. https://doi.org/10.1016/J.JCIS.2015.05.045
Sharma JK, Akhtar MS, Ameen S et al (2015) Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J Alloys Compd 632:321–325. https://doi.org/10.1016/J.JALLCOM.2015.01.172
Naika HR, Lingaraju K, Manjunath K et al (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9:7–12. https://doi.org/10.1016/J.JTUSCI.2014.04.006
Sankar R, Manikandan P, Malarvizhi V et al (2014) Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim Acta A Mol Biomol Spectrosc 121:746–750. https://doi.org/10.1016/J.SAA.2013.12.020
Ankamwar B, Damle C, Ahmad A, Sastry M (2005) Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol 5:1665–1671. https://doi.org/10.1166/JNN.2005.184
Barhoum A, García-Betancourt ML, Jeevanandam J et al (2022) Review on natural, incidental, bioinspired, and engineered nanomaterials: history, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomater 12:177. https://doi.org/10.3390/NANO12020177
Arumai Selvan D, Mahendiran D, Senthil Kumar R, Kalilur Rahiman A (2018) Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: phytochemical, antioxidant and in vitro cytotoxicity studies. J Photochem Photobiol B Biol 180:243–252. https://doi.org/10.1016/j.jphotobiol.2018.02.014
Ginting B, Maulana I, Karnila I (2020) Biosynthesis copper nanoparticles using Blumea balsamifera leaf extracts: characterization of its antioxidant and cytotoxicity activities. Surfaces and Interfaces 21:100799. https://doi.org/10.1016/J.SURFIN.2020.100799
Fathi G, Salah EL, Abdelkrim R, et al. (2022) UV-Visible spectroscopic technique for prediction of total polyphenol contents for a bunch of medicinal plant extracts.. Preprint (Version 1) available at Research Square. https://doi.org/10.21203/rs.3.rs-1370198/v1
Hammani S, Barhoum A, Nagarajan S, Bechelany M (2019) Toner waste powder (twp) as a filler for polymer blends (LDPE/HIPS) for enhanced electrical conductivity. Materials (Basel) 12.https://doi.org/10.3390/ma12193062
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/ABIO.1996.0292
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302. https://doi.org/10.1021/JF0502698
Bozin B, Mimica-Dukic N, Samojlik I et al (2008) Phenolics as antioxidants in garlic (Allium sativum L., Alliaceae). Food Chem 111:925–929. https://doi.org/10.1016/J.FOODCHEM.2008.04.071
Serçe A, Toptancı BÇ, Tanrıkut SE et al (2016) Assessment of the antioxidant activity of Silybum marianum seed extract and its protective effect against DNA oxidation, protein damage and lipid peroxidation. Food Technol Biotechnol 54:455–461. https://doi.org/10.17113/ftb.54.04.16.4323
Barhoum A, Rahier H, Benelmekki M, Assche G Van (2018) Recent trends in nanostructured particles: synthesis, functionalization, and applications. In: Fundamentals of Nanoparticles. Elsevier, pp 605–639. https://doi.org/10.1016/B978-0-323-51255-8.00024-0
Zayyoun N, Bahmad L, Laânab L, Jaber B (2016) The effect of pH on the synthesis of stable Cu2O/CuO nanoparticles by sol–gel method in a glycolic medium. Appl Phys A Mater Sci Process 122:1–6. https://doi.org/10.1007/S00339-016-0024-9/FIGURES/6
Barhoum A, Favre T, Sayegh S et al (2021) 3D self-supported nitrogen-doped carbon nanofiber electrodes incorporated Co/CoOx nanoparticles: application to dyes degradation by electro-Fenton-based process. Nanomater 11:2686. https://doi.org/10.3390/NANO11102686
Barhoum A, Van Assche G, Makhlouf ASH et al (2015) A green, simple chemical route for the synthesis of pure nanocalcite crystals. Cryst Growth Des 15:573–580. https://doi.org/10.1021/cg501121t
Sinha T, Ahmaruzzaman M (2015) Green synthesis of copper nanoparticles for the efficient removal (degradation) of dye from aqueous phase. Environ Sci Pollut Res Int 22:20092–20100. https://doi.org/10.1007/S11356-015-5223-Y
Khan M, Khan M, Adil SF et al (2013) Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine 8:1507–1516. https://doi.org/10.2147/IJN.S43309
Sastry ABS, Karthik Aamanchi RB, Prasad SRL, C, Murty BS, (2013) Large-scale green synthesis of Cu nanoparticles. Environ Chem Lett 11:183–187. https://doi.org/10.1007/S10311-012-0395-X/FIGURES/3
Mageshwari K, Sathyamoorthy R (2013) Flower-shaped CuO nanostructures: synthesis, characterization and antimicrobial activity. J Mater Sci Technol 29:909–914. https://doi.org/10.1016/j.jmst.2013.04.020
Chokkareddy R, Redhi GG (2018) Green synthesis of metal nanoparticles and its reaction mechanisms. Macabresque Hum Viol Hate Genocide, Mass Atrocity Enemy-Making 113–139.https://doi.org/10.1002/9781119418900.CH4
Chinnaiah K, Maik V, Kannan K et al (2022) Experimental and theoretical studies of green synthesized Cu2O nanoparticles using Datura Metel L. J Fluoresc 1:1–10. https://doi.org/10.1007/S10895-021-02880-4/FIGURES/7
Kumar B, Smita K, Debut A, Cumbal L (2020) Andean Sacha Inchi (Plukenetia Volubilis L.) leaf-mediated synthesis of Cu 2 O nanoparticles: a low-cost approach. Bioeng (Basel, Switzerland) 7:1–10. https://doi.org/10.3390/BIOENGINEERING7020054
Ananda Murthy HC, Zeleke TD, Tan KB et al (2021) Enhanced multifunctionality of CuO nanoparticles synthesized using aqueous leaf extract of Vernonia amygdalina plant. Results Chem 3:100141. https://doi.org/10.1016/J.RECHEM.2021.100141
Fuku X, Modibedi M, Mathe M (2020) Green synthesis of Cu/Cu2O/CuO nanostructures and the analysis of their electrochemical properties. SN Appl Sci 2:1–15. https://doi.org/10.1007/S42452-020-2704-5/FIGURES/9
Yang Y, Xu D, Wu Q (2016) Diao P (2016) Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction. Sci Reports 61(6):1–13. https://doi.org/10.1038/srep35158
Strehlow WH, Cook EL (1973) Compilation of energy band gaps in elemental and binary compound semiconductors and insulators. J Phys Chem Ref Data 2:163–200. https://doi.org/10.1063/1.3253115
Barhoum A, Melcher J, Van Assche G, et al (2017) Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: porous microparticles versus nonporous nanoparticles. J Mater Sci 52:2746–2762. https://doi.org/10.1007/s10853-016-0567-3
Kulkarni SA, Sawadh PS, Palei PK, Kokate KK (2014) Effect of synthesis route on the structural, optical and magnetic properties of Fe3O4 nanoparticles. Ceram Int 40:1945–1949. https://doi.org/10.1016/J.CERAMINT.2013.07.103
Min X, Yang Q, Zhou P (2021) Effects of nano-copper oxide on antioxidant function of copper-deficient Kazakh sheep. Biol Trace Elem Res 1–8.https://doi.org/10.1007/S12011-021-02975-W/TABLES/5
Ijaz F, Shahid S, Khan SA et al (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res 16:743–753. https://doi.org/10.4314/tjpr.v16i4.2
Peddi P, Ptsrk PR, Rani NU, Tulasi SL (2021) Green synthesis, characterization, antioxidant, antibacterial, and photocatalytic activity of Suaeda maritima (L.) Dumort aqueous extract-mediated copper oxide nanoparticles. J Genet Eng Biotechnol 19. https://doi.org/10.1186/S43141-021-00229-9
Udayabhanu NPC, Pavan Kumar MA et al (2015) Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties. Mater Sci Semicond Process 33:81–88. https://doi.org/10.1016/J.MSSP.2015.01.034
Ouidad A, Sara C, Samir D (2020) Biological properties and acute toxicity study of copper oxide nanoparticles prepared by aqueous leaves extract of Portulaca oleracea (L). Asian J Pharm Res 10:89. https://doi.org/10.5958/2231-5691.2020.00017.9
Funding
Open Access funding provided by the IReL Consortium Ahmed Barhoum (NanoStruc Research Group, Helwan University, Project PIs) would like to thank the Egypt–France Joint Driver (Imhotep, Project No. 43990SF, 2020–2022), Joint Egyptian Japanese Scientific Cooperation (JEJSC, Project No. 42811, 2021–2022), and Irish Research Council (Project ID: GOIPD/2020/340) for financial support. Thanks are given to Dublin City University (DCU) along with the Irish Research eLibrary (IReL) for providing an open access publishing agreement (1 Apr 2021–31 Dec 2024) with Springer Nature.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.S.E, and A.B.; methodology, B.D, L.S.E, B.A., A.N., and A.B.; investigation, B.D, L.S.E, B.A., A.N., and A.B.; resources, B.D, L.S.E, B.A., A.N., and A.B.; data curation, B.D, L.S.E, B.A., A.N., and A.B.; writing—original draft preparation, B.D, L.S.E, B.A., A.N., and A.B., writing—review and editing, B.D, L.S.E, B.A., A.N., and A.B.; supervision, L.S.E, and A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Copper mixed oxides (CuO/Cu2ONPs) were prepared from the extract of Phoenix dactylifera L.
• Crystallinity, particle size, and particle shape were tunned by changing Cu2+ ion concentration.
• Highest antioxidant activity was observed for 55 nm CuO/Cu2O NPs with a phase ratio of 1:1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Djamila, B., Eddine, L.S., Abderrhmane, B. et al. In vitro antioxidant activities of copper mixed oxide (CuO/Cu2O) nanoparticles produced from the leaves of Phoenix dactylifera L. Biomass Conv. Bioref. 14, 6567–6580 (2024). https://doi.org/10.1007/s13399-022-02743-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02743-3