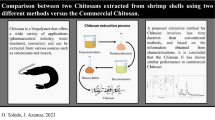
Abstract
Chitosan derivatives with lower Mw were prepared by chemical depolymerization using hydrochloric acid. This modification improves functional and biological properties of shrimp chitosan and facilitates its utilizations. The obtained chitosan depolymerization products (CDP) were characterized in terms of molecular weight (Mw), degrees of acetylation (DA) and polymerization (DP), solubility, viscosity, crystallinity, and FTIR spectroscopy. High Mw-CDP (from 94.10 to 396.46 kDa) and low Mw-CDP (< 4.4 kDa), with a DP up to 7, were withdrawn at different hydrolysis times. It is clearly demonstrated that the viscosity, the DA, and the crystallinity decreased upon depolymerization, especially in low Mw-CDP, while solubility in water and acetic acid was highly improved. FTIR analysis showed similar spectra of chitosan and CDP. The antibacterial, antifungal, and antioxidant properties of CDP were investigated and demonstrated that they were related to its Mw. Indeed, as compared to chitosan, high Mw derivatives, especially C120, possess higher antibacterial and antifungal potentials. While, low Mw-CDP, especially H120, exhibited the highest antioxidant properties. Interestingly, the used chemical depolymerization process seems to be an efficient, simple, and easy method to produce bioactive chitosan derivatives with attractive characteristics to be applied in an industrial scale, especially as functional-food components.

Graphical Abstract



Similar content being viewed by others
References
Avelelas F, Horta A, Pinto LFV, Marques SC, Nunes PM, Pedrosa R, Leandro SM (2019) Mar. Drugs. 17, 239.
Sánchez Á, Mengíbar M, Rivera-Rodríguez G, Moerchbacher B, Acosta B, Heras A (2017) Carbohydr. Polym. 157, 251–257.
Dash M, Chiellini F, Ottenbrite RM, Chiellini E (2011) Prog. Polym. Sci. 36, 981–1014.
Rajoka MSR, Zhao L, Mehwish HM, Wu Y, Mahmood S (2019) Appl. Microbiol. Biotechnol. 103, 1557–1571.
Puvvada YS, Vankayalapati S, Sukhavasi S (2012) Int. Curr. Pharm. J. 1, 258–263.
Affes S, Maalej H, Aranaz I, Acosta N, Kchaou H, Heras Á, Nasri M (2020) Carbohydr. Polym. 236, 116063.
Affes S, Nasri R, Li S, Thami T, Lee AVD, Nasri M, Maalej H (2021) Carbohydr. Polym. 255, 117341.
Santos-Moriano P, Kidibule PE, Alleyne E, Ballestros AO, Heras A, Fernandez-Lobato M, Plou FJ (2018) Process Biochem. 73, 102–108.
Kim SK, Rajapakse (2005) Carbohydr. Polym. 62, 357–368.
Santoso J, Adiputra KC, Soerdirga C, Tarman K (2020) IOP Conference Series: Earth and Environmental Science (EES), 414, 012021.
Yuan X, Zheng J, Jiao S, Cheng G, Feng G, Du Y, Liu H (2019) Carbohydr. Polym. 220, 60–70.
Li K, Xing R, Liu S, Li R, Qin Y, Meng X, Li P (2012) Carbohydr. Polym. 88, 896–903.
Younes I, Sellimi S, Rinaudo M, Jellouli K, Nasri M (2014) Int. J. Food Microbiol. 185, 57–63.
Zaeni A, Safitri E, Fuadah B, Sudiana IN (2017) J. Phys. Conf. Ser. 846, 012011.
Kasaai R, Arul J, Charlet G (2008) Ultrason Sonochem. 15, 1001–1008.
Affes S, Maalej H, Aranaz I, Acosta N, Heras Á, Nasri M (2020) Int. J. Biol. Macromol. 144, 279–288.
Song YS, Seo DJ, Jung WJ (2019) Microb. Pathog. 129, 277–283.
Wang Y, Peigen Z, Yu J, Pan X, Wang P, Lan W, Tao S (2007) Asia Pac J Clin Nutr. 16, 174–177.
Pan M, Li J, Lv X, Du G, Liu L (2019) Enzyme Microb Tech. 124, 54–62.
Zhou J, Dai R, Wang Y, Li M, Zhu Y, Chen L, Kang L, Liu Z, Yang Y, Yuan S (2019) Carbohydr. Polym. 207, 729–736.
Laokuldilok T, Potivas T, Kanha N, Surawang S, Seesuriyachan P, Wangtueai S, Phimolsiripol Y, Regenstein JM (2017) Food Biosci. 18, 28–33.
Lee DX, Xia WS, Zhang JL (2008) Food Chem. 111, 291–295.
Tømmeraas K, Vårum KM, Christensen BE, Smidsrød O (2001) Carbohydr. Res. 333, 137–44.
Aljbour ND, Beg DH, Gimbun J (2019) Chem. Eng. Technol. 42, 1741–1746.
Cabrera JC, Van Cutsem P (2005) Biochem. Eng. J. 25, 165–172.
Affes S, Aranaz I, Hamdi M, Acosta N, Ghorbel-Bellaaj O, Heras Á (2019) Int. J. Biol. Macromol. 139, 558–569.
Muzzarelli RA, Rocchetti R, Stanic V, Weckx M (1997) in: R.A.A. Muzzarelli, M.G. Peter (Eds.), Chitin Handbook Grottammare, Atec, 109–119.
Rinaudo M, Milas M, Dung PL (1993) Int. J. Biol. Macromol. 15, 281–285.
Fernandez-Kim SO (2004) Graduate Faculty of Seoul National University, Dissertation of Master of Science, 107.
Focher B, Beltrame PL, Naggi A, Tori G (1990) Carbohydr. Polym., 12, 405–418.
Vanden Berghe DA, Vlietinck AJ (1991) Meth. Plant Biochem. 6, 47–69.
Farag RS, Daw ZY, Hewedi FM, El-baroty GSA (1989) J. Food Prot. 52, 665–667.
Bersuder P, Hole M, Smith G (1998) J. Am. Oil Chem. Soc. 75, 181–187.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Free Radic. Biol. Med. 26, 1231–1237.
Yildirim A, Mavi A, Kara AA (2001) J. Agric. Food Chem. 49, 4083–4089.
Prieto P, Pineda M, Aguilar M (1999) Anal Biochem. 269, 337–341.
Kasaai MR, Arul J, Charlet G (2013) Sci. World J. 2013, 11 pages.
Li D-D, Tao Y, Shi Y-N, Han Y-N, Yang N, Xu X-M (2020) Food Chem. 309, 125767.
Vårum KM, Ottøy MH, Smidsrøda O (2001) Carbohydr. Polym. 46, 89–98.
Gonçalves C, Ferreira N, Louenço L (2021) Polymers 13, 2466.
Li J, Du Y, Yang J, Feng T, Li A, Chen P (2005) Polym. Degrad. Stab. 87, 441–448.
Affes S, Aranaz I, Acosta N, Heras Á, Nasri M (2021) Int. J. Biol. Macromol. 182, 730–742.
Asli A, Brouillette E, Ster C, Ghinet MG, Brzezinsid R, Lacasse P, Jacques M, Malouin F (2017) PLoS One 12, e0176988.
Uchida Y, Izume M, Ohtakara A In Braek GS, Anthonsen T, Sandford P (1989) Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications. 372–382.
Li XF, Feng XQ, Yang S, Fu GQ, Wang TP, Su ZX (2010) Carbohydr. Polym. 79, 493–499.
Jeon YJ, Park PJ, Kim SK (2001) Carbohydr. Polym. 44, 71–76.
Ueno K, Yamaguchi T, Sakairi N, Nishi N, Tokura S (1997) In A. Domard, G. A. F. Roberts, & K. M. Varum, Advances in Chitin Science. 156–161.
Shahidi F, Arachchi JKV, Jeon YJ (1999) Trends Food Sci. Technol. 10, 37–51.
Vishu Kumar AB, Varadaraj MC, Gowda LR, Tharanathan RN (2007) BBA-Gen. Subjects. 1770, 495–505.
Hamdi M, Hajji S, Affes S, Taktak W, Maalej H, Nasri M, Nasri R (2018) Food Hydrocoll. 77, 534-548.
Rahman MH, Hjeljord LG, Aam BB, Sørlie M, Tronsmo A (2014) Eur. J. Plant Pathol. 141, 147–158.
Chang CT, Lin YL, Lu SW, Huang CX, Wang YT, Chung YC (2016) PLoS One. 11, 1–17.
Huang J, Zhao D, Hu S, Mao J, Mei L (2012) Carbohydr. Polym. 87, 2231–2236.
Funding
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia. The financial support for this study is provided by the Spanish Ministry of Economy and Competitiveness [Grants MAT2015-65184-C2-1-R].
Author information
Authors and Affiliations
Contributions
Sawsan Affes: conceptualization, methodology, validation, formal analysis, investigation, writing-original draft. Inmaculada Aranaz: conceptualization, resources, writing-review and editing, investigation. Niuris Acosta: resources, visualization, writing-review and editing. Angeles Heras: resources, visualization, writing-review and editing. Moncef Nasri: supervision, resources, visualization, writing-review and editing. Hana Maalej: supervision, conceptualization, resources, writing-review and editing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chitosans with varying molecular weight were prepared by chemical depolymerization.
• Physiochemical characteristics of chitosan derivatives were determined.
• Antimicrobial and antioxidant potentials of chitosan derivatives were evaluated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Affes, S., Aranaz, I., Acosta, N. et al. Physicochemical and biological properties of chitosan derivatives with varying molecular weight produced by chemical depolymerization. Biomass Conv. Bioref. 14, 4111–4121 (2024). https://doi.org/10.1007/s13399-022-02662-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02662-3