Abstract
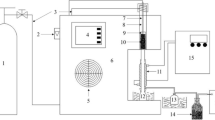
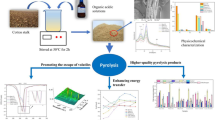
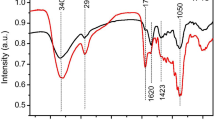
In the present study, the effects of pretreatments with different solvents (hydrochloric acid, sodium hydroxide, and ethanol) on the physicochemical structure and pyrolysis behavior of tobacco stem (TS) were investigated. Elemental compositions analysis showed that acid washing increased the carbon content and exhibited a high removal percentage for the inorganic species. SEM, BET, and FTIR analysis showed that the pretreatments exerted different effect on the surface and microstructure. Furthermore, the pyrolysis characteristics analysis indicated that solvent pretreatment increased the initial pyrolysis temperature due to the removal of unstable volatile components, especially for the acid and alkali washing samples. Meanwhile, hydrochloric acid pretreatment increased the maximum pyrolysis rate temperature of cellulose decomposition stage and decreased the char residues due to the large removal of inorganic species, while alkali treatment produced the opposite result. Pyrolysis kinetic analysis based on Coats-Redfern method showed that F1.5 chemical reaction model can be used to describe the pyrolysis stages of TS samples and a decreased activation energy was observed during the decomposition of cellulose stage for the acid and alkali washed samples. The released gaseous products during the pyrolysis process were also monitored by using TG-FTIR technique, and the gaseous release behavior was different from each other.








Similar content being viewed by others
References
Wu W, Mei Y, Zhang L, Liu R, Cai J (2015) Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 156:71–80. https://doi.org/10.1016/j.fuel.2015.04.016
Gao W, Chen K, Xiang Z, Yang F, Zeng P, Li J, Yang R, Rao G, Tao H (2013) Kinetic study on pyrolysis of tobacco residues from the cigarette industry. Ind Crop Prod 44:152–157. https://doi.org/10.1016/j.indcrop.2012.10.032
Carlson TR, Cheng YT, Jae J, Huber GW (2011) Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ Sci 4(1):145–161. https://doi.org/10.1039/c0ee00341g
Zhang L, Liu R, Yin R, Mei Y (2013) Upgrading of bio-oil from biomass fast pyrolysis in China: a review. Renew Sustain Energy Rev 24:66–72. https://doi.org/10.1016/j.rser.2013.03.027
Carpenter D, Westover TL, Czernik S, Jablonski W (2014) Biomass feedstocks for renewable fuel production: a review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem 16(2):384–406. https://doi.org/10.1039/c3gc41631c
Paulsen AD, Hough BR, Williams CL, Teixeira AR, Schwartz DT, Pfaendtner J, Dauenhauer PJ (2014) Fast pyrolysis of wood for biofuels: spatiotemporally resolved diffuse reflectance in situ spectroscopy of particles. Chemsuschem 7(3):765–776. https://doi.org/10.1002/cssc.201301056
Talmadge MS, Baldwin RM, Biddy MJ, McCormick RL, Beckham GT, Ferguson GA, Czernik S, Magrini-Bair KA, Foust TD, Metelski PD et al (2014) A perspective on oxygenated species in the refinery integration of pyrolysis oil. Green Chem 16(2):407–453. https://doi.org/10.1039/c3gc41951g
Abdullah H, Wu H (2009) Biochar as a fuel: 1. Properties and grindability of biochars produced from the pyrolysis of Mallee wood under slow-heating conditions. Energy Fuel 23(8):4174–4181. https://doi.org/10.1021/ef900494t
Jung C, Park J, Lim KH, Park S, Heo J, Her N, Oh J, Yun S, Yoon Y (2013) Adsorption of selected endocrine disrupting compounds and pharmaceuticals on activated biochars. J Hazard Mater 263:702–710. https://doi.org/10.1016/j.jhazmat.2013.10.033
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Manya JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46(15):7939–7954. https://doi.org/10.1021/es301029g
Lehmann J (2007) A handful of carbon. Nature 447(7141):143–144. https://doi.org/10.1038/447143a
Lin Y, Yan W, Sheng K (2016) Effect of pyrolysis conditions on the characteristics of biochar produced from a tobacco stem. Waste Manag Res 34(8):793–801. https://doi.org/10.1177/0734242x16654977
Bushra B, Remya N (2020) Biochar from pyrolysis of rice husk biomass-characteristics, modification and environmental application. Biomass Convers Biorefinery: 1-12. https://doi.org/10.1007/s13399-020-01092-3
Chen R, Zhang J, Lun L, Li Q, Zhang Y (2019) Comparative study on synergistic effects in co-pyrolysis of tobacco stalk with polymer wastes: thermal behavior, gas formation, and kinetics. Bioresour Technol 292:1–10. https://doi.org/10.1016/j.biortech.2019.121970
Strezov V, Popovic E, Filkoski RV, Shah P, Evans T (2012) Assessment of the thermal processing behavior of tobacco waste. Energy Fuel 26(9):5930–5935. https://doi.org/10.1021/ef3006004
Gomez-Siurana A, MarcillaA BM, Berenguer D, Martinez-Castellanos I, Menargues S (2013) TGA/FTIR study of tobacco and glycerol-tobacco mixtures. Thermochim Acta 573:146–157. https://doi.org/10.1016/j.tca.2013.09.007
Chen C, Luo Z, Yu C, Wang T, Zhang H (2017) Transformation behavior of potassium during pyrolysis of biomass. RSC Adv 7(50):31319–31326. https://doi.org/10.1039/c7ra05162j
Taherzadeh MJ, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9(9):1621–1651. https://doi.org/10.3390/ijms9091621
Yao L, Yoo CG, Pu Y, Meng X, Muchero W, Tuskan GA, Tschaplinski TJ, Ragauskas AJ, Yang H (2019) Physicochemical changes of cellulose and their influences on Populus trichocarpa digestibility after different pretreatments. BioResources 14(4):9658–9676. https://doi.org/10.15376/biores.14.4.9658-9676
Jiang L, Hu S, Xiang J, Su S, Sun L, Xu K, Yao Y (2012) Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour Technol 116:278–284. https://doi.org/10.1016/j.biortech.2012.03.051
Chen D, Cen K, Chen F, Ma Z, Zhou J, Li M (2020) Are the typical organic components in biomass pyrolyzed bio-oil available for leaching of alkali and alkaline earth metallic species (AAEMs) from biomass? Fuel 260:116347. https://doi.org/10.1016/j.fuel.2019.116347
Cen K, Zhang J, Ma Z, Chen D, Zhou J, Ma H (2019) Investigation of the relevance between biomass pyrolysis polygeneration and washing pretreatment under different severities: water, dilute acid solution and aqueous phase bio-oil. Bioresour Technol 278:26–33. https://doi.org/10.1016/j.biortech.2019.01.048
Chen Z, Leng E, Zhang Y, Zheng A, Peng Y, Gong X, Huang Y, Qiao Y (2018) Pyrolysis characteristics of tobacco stem after different solvent leaching treatments. J Anal Appl Pyrolysis 130:350–357. https://doi.org/10.1016/j.jaap.2017.12.009
Sari NH, Wardana ING, Irawan YS, Siswanto E (2018) Characterization of the chemical, physical, and mechanical properties of NaOH-treated natural cellulosic fibers from corn husks. J Nat Fibers 15(4):545–558. https://doi.org/10.1080/15440478.2017.1349707
Dalle D, Hansen B, Zattera AJ, Francisquetti EL, Catto AL, Borsoi C (2021) Kinetic evaluation of tobacco stalk waste exposed to alkaline surface treatment under different conditions. Cellulose 28(4):2053–2073. https://doi.org/10.1007/s10570-020-03657-x
Cao Y, Zhou H, Fan J, Zhao H, Zhou T, Hack P, Chan C, Liou J, Pan W (2008) Mercury emissions during cofiring of sub-bituminous coal and biomass (chicken waste, wood, coffee residue, and tobacco stalk) in a laboratory-scale fluidized bed combustor. Environ Sci Technol 42(24):9378–9384. https://doi.org/10.1021/es8016107
Fan Y, Li L, Tippayawong N, Xia S, Cao F, Yang X, Zhao Z, Li H (2019) Quantitative structure-reactivity relationships for pyrolysis and gasification of torrefied xylan. Energy 188:116119. https://doi.org/10.1016/j.energy.2019.116119
White JE, Catallo WJ, Legendre BL (2011) Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J Anal Appl Pyrolysis 91(1):1–33. https://doi.org/10.1016/j.jaap.2011.01.004
Cai J, Li B, Chen C, Wang J, Zhao M, Zhang K (2016) Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour Technol 220:305–311. https://doi.org/10.1016/j.biortech.2016.08.098
Liang M, Yang T, Zhang G, Zhang K, Wang L, Li R, He Y, Wang J, Zhang J (2021) Effects of hydrochloric acid washing on the structure and pyrolysis characteristics of tobacco stalk. Biomass Convers Biorefinery 1-14. https://doi.org/10.1007/s13399-021-01616-5
Wang C, Li L, Chen R, Ma X, Lu M, Ma W, Peng H (2019) Thermal conversion of tobacco stem into gaseous products. J Therm Anal Calorim 137(3):811–823. https://doi.org/10.1007/s10973-019-08010-4
Kuang M, Li Z (2014) Review of gas/particle flow, coal combustion, and NOx emission characteristics within down-fired boilers. Energy 69:144–178. https://doi.org/10.1016/j.energy.2014.03.055
Reza MT, Yang X, Coronella CJ, Lin H, Hathwaik U, Shintani D, Neupane BP, Miller GC (2016) Hydrothermal carbonization (HTC) and pelletization of two arid land plants bagasse for energy densification. ACS Sustain Chem Eng 4(3):1106–1114. https://doi.org/10.1021/acssuschemeng.5b01176
Abedi A, Cheng H, Dalai AK (2018) Effects of natural additives on the properties of sawdust fuel pellets. Energy Fuel 32(2):1863–1873. https://doi.org/10.1021/acs.energyfuels.7b03663
Gao P, Zhou Y, Meng F, Zhang Y, Liu Z, Zhang W, Xue G (2016) Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 97:238–245. https://doi.org/10.1016/j.energy.2015.12.123
Oh GH, Yun CH, Park CR (2003) Role of KOH in the one-stage KOH activation of cellulosic biomass. Carbon Lett 4(4):180–184. https://doi.org/10.1016/j.bmcl.2014.09.027
Liang M, Zhang K, Lei P, Wang B, Shu C, Li B (2020) Fuel properties and combustion kinetics of hydrochar derived from co-hydrothermal carbonization of tobacco residues and graphene oxide. Biomass Convers Biorefinery 10(1):189–201. https://doi.org/10.1007/s13399-019-00408-2
Shimada N, Kawamoto H, Saka S (2008) Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J Anal Appl Pyrolysis 81(1):80–87. https://doi.org/10.1016/j.jaap.2007.09.005
Jiang L, Hu S, Sun L, Su S, Xu K, He L, Xiang J (2013) Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour Technol 146:254–260. https://doi.org/10.1016/j.biortech.2013.07.063
Le Brech Y, Ghislain T, Leclerc S, Bouroukba M, Delmotte L, Brosse N, Snape C, Chaimbault P, Dufour A (2016) Effect of potassium on the mechanisms of biomass pyrolysis studied using complementary analytical techniques. Chemsuschem 9(8):863–872. https://doi.org/10.1002/cssc.201501560
Nowakowski DJ, Jones JM, Brydson RMD, Ross AB (2007) Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 86(15):2389–2402. https://doi.org/10.1016/j.fuel.2007.01.026
Ma Z, Chen D, Gu J, Bao B, Zhang Q (2015) Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods. Energy Convers Manag 89:251–259. https://doi.org/10.1016/j.enconman.2014.09.074
Chen D, Cen K, Cao X, Chen F, Zhang J, Zhou J (2021) Insight into a new phenolic-leaching pretreatment on bamboo pyrolysis: release characteristics of pyrolytic volatiles, upgradation of three phase products, migration of elements, and energy yield. Renew Sustain Energy Rev 136:110444. https://doi.org/10.1016/j.rser.2020.110444
Zhou S, Xu YB, Wang CH, Tian ZF (2011) Pyrolysis behavior of pectin under the conditions that simulate cigarette smoking. J Anal Appl Pyrolysis 91(1):232–240. https://doi.org/10.1016/j.jaap.2011.02.015
Yuan T, Tahmasebi A, Yu J (2015) Comparative study on pyrolysis of lignocellulosic and algal biomass using a thermogravimetric and a fixed-bed reactor. Bioresour Technol 175:333–341. https://doi.org/10.1016/j.biortech.2014.10.108
Baker RR (1975) The formation of the oxides of carbon by the pyrolysis of tobacco. Btrge Zur Tabakforschung 8(1):16–27. https://doi.org/10.2478/cttr-2013-0350
Lu Q, Zhang Z, Yang X, Dong C, Zhu X (2013) Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: analytical Py-GC/MS study. J Anal Appl Pyrolysis 104:139–145. https://doi.org/10.1016/j.jaap.2013.08.011
Pu G, Zhu W, Zhou H, Liu Y, Zhang Z (2015) Kinetics of co-gasification of low-quality lean coal and biomass. BioResources 10(2):2773–2782. https://doi.org/10.15376/biores.10.2.2773-2782
Funding
This work was supported by the the project (No. AW201922) from China Tobacco Henan Industrial Co., Ltd. and the Research Foundation (No. 2019ZCKJ304, 2014BSJJ067) of Zhengzhou University of Light Industry.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The summary is as follows: Conceptualization: Miao Liang. Material preparation: Ming Han. Methodology: Xiao Li. Formal analysis and investigation: Qingqing Zhao, Wei Wang, Xuhe Wei. Writing—original draft preparation: Xiao Li, Qingqing Zhao. Writing—review and editing: Zhongya Guo, Miao Liang, Ke Zhang , Bin Li. Funding acquisition: Xiao Li, Ming Han. Supervision: Miao Liang, Zhongya Guo. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Zhao, Q., Han, M. et al. Pyrolysis characteristics and kinetic analysis of tobacco stem pretreated with different solvents. Biomass Conv. Bioref. 14, 501–515 (2024). https://doi.org/10.1007/s13399-021-02280-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02280-5