Abstract
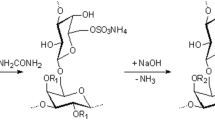
Arabinogalactan is a biologically active water-soluble polysaccharide contained in great amounts in larch wood. A sulfate group introduced into the arabinogalactan molecule increases its biological activity and imparts new anticoagulant and hypolipedemic properties. A new method for the catalytic sulfation of arabinogalactan with ammonium sulfamate is proposed. The catalytic activity in the reaction of arabinogalactan sulfation with ammonium sulfamate has been investigated for both the base activators (urea and thiourea) and oxidants (KMnO4 and K2Cr2O7). It has been shown that, in this process, the most effective activator is KMnO4, which ensures the highest (11.3 wt %) sulfur content in sulfated arabinogalactan. The effect of temperature and time of the arabinogalactan sulfation with ammonium sulfamate and the nature of solvents used on the sulfur content in sulfated arabinogalactan has been examined. It has been found that the highest (11.3%) sulfur content in the product is obtained by sulfation of arabinogalactan in 1,4-dioxane at a temperature of 90 °C for 4 h with the KMnO4 activator. The introduction of a sulfate group into the arabinogalactan molecule has been confirmed by the FTIR and NMR spectroscopy data. The FTIR spectrum of sulfated arabinogalactan, in contrast to that of the initial arabinogalactan, includes an absorption band at 1249 cm−1. According to the 13C NMR spectroscopy data, the sulfate groups are located at carbon atoms C2 and C4 of the main galactan chain and at carbon atom C6 of the end galactose units of the main and side arabinogalactan chains. It has been established by gel permeation chromatography that, during the sulfation, the molecular mass of arabinogalactan molecules increases proportionally to the introduced sulfate groups. The absence of low molecular weight fractions is indicative of the absence of depolymerization under the conditions of sulfation and evolution of sulfated arabinogalactan.










Similar content being viewed by others
Data availability
N/A.
Code availability
N/A.
References
Medvedeva EN, Babkin VA, Ostroukhova LA (2003) Arabinogalactan from larch - properties and prospects of use (review). Chem raw plant mater 1:27–27
G.O. Aspinall, Some recent developments in the chemistry of arabinogalactans, Chim. Biochim. Lign., Cellul. Hemicell. Actes du Symposium International de Grenoble. (1964). 89–97.
Arifkhodzhaev AO (2000) Galactans and galactan-containing polysaccharides of higher plants. Chem nat comp 3:185–197
Medvedeva SA, Alexandrova GP, Tantsyrev AP (2002) Gel permeation chromatography of arabinogalactan. Izv univer Forest J 6:108–114
Karacsonyi S, Kovacik V, Alfoldi J, Kubackova M (1984) Chemical and 13C studies of arabinogalactan from Larix sibirica L. Carbohydr Res 134:265–274
Babkin VA, Neverova NA, Medvedeva EN, Fedorova TE, Levchuk AA (2016) Investigation of physicochemical properties of arabinogalactan of different larch species. Russ J Bioorg Chem 42:707–711
Ponder GR, Richards GN (1997) Arabinogalactan from western larch, part II; a reversible order-disorder transition. J Carbohydr Chem 16:195–211
Ponder GR, Richards GN (1997) Arabinogalactan from western larch, part III; alkaline degradation revisited, with novel conclusions on molecular structure. Carbohydr Polym 34:251–261
Goellner EM, Utermoehlen J, Kramer R, Classen B (2011) Structure of arabinogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinogalactans. Carbohydr Polym 86:1739–1744
Ehrenfreund-Kleinman T, Azzam T, Falk R, Golenser J, Domb AJ (2002) Synthesis and characterization of novel water soluble amphotericin B – arabinogalactan conjugates. Biomaterials 23(5):1327–1335
Mucalo MR, Bullen CR, Manley-Harris M, McIntire TM (2002) Arabinogalactan from the Western larch tree: a new, purified and highly water-soluble polysaccharide-based protecting agent for maintaining precious metal nanoparticles in colloidal suspensions. J Mater Sci 37(3):493–504
Medvedeva SA, Alexandrova GP, Grishchenko LA, Tyukavkina NA (2002) Synthesis of iron (II, III) -containing derivatives of arabinogalactan. J Gen Chem (Rus) 72(9):1569–1573
Merce ALR, Landaluze JS, Mangrich AS, Szpoganicz B, Sierakowski MR (2001) Complexes of arabinogalactan of Pereskia aculeata and Co2+, Cu2+, Mn2+ and Ni2+. Bioresour Technol 76(1):29–37
Usov AI, Bilan MI (2009) Fucoidans — sulfated polysaccharides of brown algae. Russ Chem Rev 78(8):785–799. https://doi.org/10.1070/RC2009v078n08ABEH004063
Adhikari U, Mateu CG, Chattopadhyay K, Pujol CA, Damonte EB, Ray B (2006) Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry 67:2474. https://doi.org/10.1016/j.phytochem.2006.05.024
Hemmingson JA, Falshaw R, Furneaux RH, Thompson K (2006) Structure and Antiviral Activity of the Galactofucan Sulfates Extracted from Undaria Pinnatifida (Phaeophyta). J Appl Phycol 18:185. https://doi.org/10.1007/s10811-006-9096-9
Yoon S-J, Pyun Y-R, Hwang J-K, Mourao PAS (2007) A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity Carbohydr. Res 342:2326. https://doi.org/10.1016/j.carres.2007.06.019
J. da Silva Barbosa, D. A. Sabry, C.H.F. Silva, D. L. Gomes, A. P. Santana-Filho, G. L. Sassaki, H. A. O. Rocha. Immunostimulatory Effect of sulfated galactans from the green seaweed Caulerpa cupressoides var. flabellata. Marine Drugs. 18(5), (2020). 234. https://doi.org/10.3390/md18050234
He M, Yang Y, Shao Z, Zhang J, Feng C, Wang L, Mao W (2021) Chemical structure and anticoagulant property of a novel sulfated polysaccharide from the green alga Cladophora oligoclada. Mar Drugs 19:554. https://doi.org/10.3390/md19100554
Kostyro YA, Stankevich VK (2015) New approach to the synthesis of an active substance of Agsular® pharmaceutical for the prevention and treatment of atherosclerosis. Rus Chem Bull 64:1576–1580
N.Yu. Vasilyeva, A.V. Levdansky, A.A. Karacharov, E.V. Mazurova, G.N. Bondarenko, V.A. Levdansky, A.S. Kazachenko, B.N. Kuznetsov Study of structure of product’s obtained by sulfation of arabinogalactan from Larch wood with chlorosulfonic acid in pyridine, J. Sib. Fed. Univ. Chem. 7 (2014) 547–555.
Kuznetsova SA, Vasilyeva NYu, Drozd NN, Mikhailenko MA, Shakhtshneider TP, Malyar YuN, Kuznetsov BN, Chesnokov NV (2020) Sulfated derivatives of arabinogalactan and their anticoagulant activity. Russ J Bioorg Chem 46:1323–1329
L. Qin, M. He, Y. Yang, Z. Fu, C. Tang, Z. Shao, J. Zhang, W/ Mao, Anticoagulant-active sulfated arabinogalactan from Chaetomorpha linum: structural characterization and action on coagulation factors, Carbohydr. Polym. 242 (2020), 116394.
Mestechkina NM, Shcherbukhin VD (2010) Sulfated polysaccharides and their anticoagulant activity: A review. Appl Biochem Microbiol 46:267–273
G. Fang, Y. Ma, Preparation of arabinogalactan sulfate from arabinogalactan. Patent CN 101054420 (2007).
Vasilyeva NYu, Levdansky AV, Kazachenko AS, Djakovitch L, Pinel C, Kuznetsov BN (2013) Sulfation of mechanically activated arabinogalactan by complex sulfuric anhydride – pyridine in pyridine medium. J Sib Fed Univ Chem 6:158–169
Ya.A. Kostyro, T.V. Ganenko, S.A. Medvedeva, B.G. Sukhov, B.A. Trofimov, Method for preparing sulfated derivatives of arabinogalactan possessing anticoagulating and hypolipidemic activity. Patent RU 2319707 (2008).
Tang S, Wang T, Huang C, Lai C, Fan Y, Yong Q (2019) Sulfated modification of arabinogalactans from Larix principis-rupprechtii and their antitumor activities. Carbohydr Polym 215:207–212
Dzhil'bert E.E. Sulfonation of organic compounds. Moscow (1969). 416 p. (In Russ.).
Benson GA, Spillane WJ (1980) Sulfamic acid and its N-substituted derivatives. Chem Rev 80(2):151–186
Kuznetsov BN, Vasilyeva NYu, Levdasky AV, Karacharov AA, Krylov AS, Mazurova EV, Bondarenko GN, Levdansky VA, Kazachenko AS (2017) The Raman spectroscopy, XRD, SEM, and AFM study of arabinogalactan sulfates obtained using sulfamic acid. Russ J Bioorg Chem 43:722–726
Vasilyeva NYu, Levdansky AV, Kuznetsov BN, Skvortsova GP, Kazachenko AS, Djakovitch L, Pinel C (2015) Sulfation of arabinogalactan by sulfamic acid in dioxane. Russ J Bioorg Chem 41:725–731
Kostyro YA, Medvedeva SA, Sukhov BG (2004) Synthesis of sulfated derivatives of arabinogalactan. Engin Tech (Rus) 3:10–12
Usov AI (2001) Problems and advances in structural analysis of sulfated polysaccharides of red algae. Chem raw plant mater 2:7–20
A. Tonani, A. Novello, K. Sirna, Cellulose substrate with anti-flame properties and relative production method. (2018) Bull. № 10 ,
M. Lewin. I. Sulfation of cotton and wool flame retarding of polymers with sulfamates, J. Fire Sci. 15 (1997) 263–267.
Medvedeva SA, Alexandrova GP, Dubrovin VI (2002) Larch arabinogalactan is a promising polymer matrix for biogenic metals. Butlerov Commun 7:45–49
Kuznetsov BN, Vasilyeva NYu, Levdansky AV, Maximov NG, Kazachenko AS, Skvortsova GP, Djakovitch L, Pinel C (2017) Synthesis and study of copper-containing polymers based on sulfated arabinogalactan. Rus J Bioorg Chem 43(7):727–731
Ya.N. Kostyro, O.A. Silizneva, A.I. Spark. Prospects for the development and use in medical practice of drugs based on heparinoids. Bull. VSNTS SB RAMS. 4 (80(2)) (2011) 249–254.
Levdansky AV, Vasilyeva NYu, Kondrasenko AA, Levdansky VA, Malyar YuN, Kazachenko AS, Kuznetsov BN (2021) Sulfation of arabinogalactan with sulfamic acid under homogeneous conditions in dimethylsulfoxide medium. Wood Sci Technol. https://doi.org/10.1007/s00226-021-01341-2
Lewin M, Brozek J, Martens MM (2002) The system polyamide/sulfamate/dipentaerythritol: flame retardancy and chemical reactions. Polym Adv Technol 13(10–12):1091–1102
Coquelle M, Duquesne S, Casetta M, Sun J, Gu X, Zhang S, Bourbigot S (2015) Flame retardancy of PA6 using a guanidine sulfamate/melamine polyphosphate mixture. Polymers 7(2):316–332
A.S. Kazachenko, N.Yu. Vasilieva, V.S. Borovkova, O.Yu. Fetisova, N. Issaoui, Yu.N. Malyar, E.V. Elsuf’ev, A.A. Karacharov, A.M. Skripnikov, A.V. Miroshnikova, A.S. Kazachenko, D.V. Zimonin, V.A. Ionin, Food xanthan polysaccharide sulfation process with sulfamic acid. Foods. 10 (2021) 2571. https://doi.org/10.3390/foods10112571
Al-Horani RA, Desai UR (2010) Chemical sulfation of small molecules—advances and challenges. Tetrahedron 66:2907–2918
Spillane W, Malaubier J-B (2014) Sulfamic acid and its N- and O-substituted derivatives. Chem Rev 114:2507–2586. https://doi.org/10.1021/cr400230c
Kazachenko AS, Malyar YN, Vasilyeva NY, Fetisova OY, Chudina AI, Sudakova IG, Antonov AV, Borovkova VS, Kuznetsova SA (2021) Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci Technol 55:1091–1107. https://doi.org/10.1007/s00226-021-01299-1
Kazachenko AS, Vasilyeva NY, Malyar YN, Kazachenko AS (2021) Mathematical optimization, the effect of the catalyst and solvent on the process of starch sulfation with sulfamic acid. Lect Notes Netw Syst 230:1–8
Kuznetsov BN, Vasilyeva NY, Kazachenko AS, Levdansky VA, Kondrasenko AA, Malyar YN, Skvortsova GP, Lutoshkin MA (2020) Optimization of the process of abies ethanol lignin sulfation by sulfamic acid–urea mixture in 1,4-dioxane medium. Wood Sci Technol 54:365–381. https://doi.org/10.1007/s00226-020-01157-6
Akman F, Issaoui N, Kazachenko AS (2020) Intermolecular hydrogen bond interactions in the thiourea/water complexes (Thio-(H2O)n) (n = 1, …, 5): X-ray, DFT, NBO, AIM, and RDG analyses. J Mol Model 26:1–16. https://doi.org/10.1007/s00894-020-04423-3
Civera C, del Valle JC, Elorza MA, Elorza B, Arias C, Díaz-Oliva C, Catalán J, Blanco FG (2020) Solvatochromism in urea/water and urea-derivative/water solutions. Phys Chem Chem Phys 22:25165–25176. https://doi.org/10.1039/d0cp03816d
Yu.N. Malyar, N.Y. Vasilyeva, A.S. Kazachenko, V.S. Borovkova, A.M. Skripnikov, A.V. Miroshnikova, D.V. Zimonin, V.A. Ionin, A.S. Kazachenko, N. Issaoui. Modification of arabinogalactan isolated from Larix sibirica Ledeb. into sulfated derivatives with the controlled molecular weights. Molecules 2021, 26, 5364.https: //doi.org/https://doi.org/10.3390/molecules26175364
A.S. Kazachenko, Y.N. Malyar, N.Y. Vasilyeva, V.S. Borovkova, N. Issaoui. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Conv. Bioref. (2021), 1–10. https://doi.org/10.1007/s13399-021-01895-y
Malyar YN, Kazachenko A, Vasilyeva NY, Fetisova OY, Borovkova V, Miroshnikova A, Levdansky A, Skripnikov A (2021) Sulfation of wheat straw soda lignin: role of solvents and catalysts. Catal Today. https://doi.org/10.1016/j.cattod.2021.07.033
Fundamentals of Electrochemistry, 2nd Edition. Vladimir S. Bagotsky (Editor) ISBN: 978–0–471–70058–6 (2005) 752 Pages.
Kong L, Yu L, Feng T, Yin X, Liu T, Dong L (2015) Physicochemical characterization of the polysaccharide from Bletilla striata: effect of drying method. Carbohydr Polym 125:1–8. https://doi.org/10.1016/j.carbpol.2015.02.042
Dang Z, Feng D, Liu X, Yang T, Guo L, Liang J, Liang J, Hu F, Cui F, Feng S (2013) Structure and antioxidant activity study of sulfated acetamido-polysaccharide from Radix Hedysari. Fitoterapia 89:20–32. https://doi.org/10.1016/j.fitote.2013.05.011
Wang J, Bao A, Meng X, Guo H, Zhang Y, Zhao Y, Kong W, Liang J, Yao J, Zhang J (2018) An efficient approach to prepare sulfated polysaccharide and evaluation of anti-tumor activities in vitro. Carbohydr Polym 184:366–375. https://doi.org/10.1016/j.carbpol.2017.12.065
Kazachenko AS, Akman F, Sagaama A, Issaoui N, Malyar YuN, Vasilieva NYu, Borovkova VS (2021) Theoretical and experimental study of guar gum sulfation. J Mol Model 27:5. https://doi.org/10.1007/s00894-020-04645-5
A.S. Kazachenko, F. Akman, M. Medimagh, N. Issaoui, N. Yu. Vasilieva, Yu. N. Malyar, I. G. Sudakova, A.A. Karacharov, A.V. Miroshnikova and O.M. Al-Dossary. Sulfation of diethylaminoethyl-cellulose: QTAIM topological analysis and experimental and DFT study of the properties. ACS Omega. https://doi.org/10.1021/acsomega.1c02570.
A.S. Kazachenko, F. Akman, Y.N. Malyar, N. Issaoui, N. Yu. Vasilieva, A.A. Karacharov, Synthesis optimization, DFT and physicochemical study of chitosan sulfates. Journal of Molecular Structure. V.1245, 2021. https://doi.org/10.1016/j.molstruc.2021.131083
A. S. Kazachenko, Yu. N. Malyar, N. Yu. Vasilyeva, G. N. Bondarenko, I. V. Korolkova, A. V. Antonov, A. A. Karacharov, O. Yu. Fetisova, G. P. Skvortsova «Green» synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Conv. Bioref. (2020). https://doi.org/10.1007/s13399-020-00855-2
Akman F, Kazachenko AS, Vasilyeva NYu, Malyar YuN (2020) Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. Journal of Molecular Strucrure. https://doi.org/10.1016/j.molstruc.2020.127899
L.J. Bellamy Advances in Infrared Group Frequencies. London: Methuen, 1968, 328 p.
Caputo HE, Straub JE, Grinstaff MW (2019) Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem Soc Rev 48(8):2338–2365
Muthukumar J, Chidambaram R, Sukumaran S (2021) Sulfated polysaccharides and its commercial applications in food industries—a review. J Food Sci Technol 58(7):2453–2466
Slyusarenko NV, Vasilyeva NYu, Kazachenko AS, Gerasimova MA, Romanchenko AS, Slyusareva EA (2020) Synthesis and properties of interpolymer complexes based on chitosan and sulfated arabinogalactan. Polym Sci, Ser B 62(3):272–278. https://doi.org/10.1134/S1560090420020050
Acknowledgements
Experimental work was conducted within the framework of the budget plan # 0287-2021-0017 for the Institute of Chemistry and Chemical Technology SB RAS using the equipment of Krasnoyarsk Regional Research Equipment Center of SB RAS. Methodology work was supported by Researchers Supporting Project number (RSP-2021/61), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
In the course of work on this article, the authors did not conduct research on animals and humans in any form.
Consent to participate
N/A.
Consent for publication
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazachenko, A.S., Vasilieva, N.Y., Malyar, Y.N. et al. Sulfation of arabinogalactan with ammonium sulfamate. Biomass Conv. Bioref. 14, 719–731 (2024). https://doi.org/10.1007/s13399-021-02250-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02250-x