Abstract
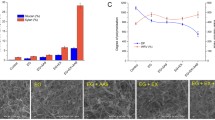
Currently, there is an increasing demand for developing enzymatic blends that can efficiently deconstruct the hemicellulose present in plant fibers, since these fibers can be further employed to build new engineered materials. Sisal fibers are investigated as a source of cellulosic material to manufacture new materials, as reinforced fiber with plastic additives. A critical step to obtain the cellulosic matrix is the removal of hemicellulose present on these fibers. In the present study, a mini-xylanosome was tailored using a miniature version of C. thermocellum’s scaffolding protein (two cohesins I domains and one carbohydrate-binding module family 3) and the xylanase XynZ. The designed xylanosome displayed higher thermal stability at 70 °C in comparison to the free form XynZ, 134 and 90 min of half-lives, respectively. Complexing xylanases into mini-xylanosomes or immobilizing complexes into cellulose did not reduce activity loss by tannic acid. Regarding sisal fiber deconstruction, the mini-xylanosome displayed better hydrolyzing capacity than the non-complexed xylanase, resulting in three times higher reducing sugars. Immobilized mini-xylanosome could be subjected to 72 h of reuse cycles at 50 and 60 °C, and at 70 °C for 48 h.







Similar content being viewed by others
References
Abraham A, Mathew AK, Sindhu R et al (2016) Potential of rice straw for bio-refining: an overview. Bioresour Technol 215:29–36. https://doi.org/10.1016/j.biortech.2016.04.011
Kim M, Day DF (2011) Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J Ind Microbiol Biotechnol 38:803–807. https://doi.org/10.1007/s10295-010-0812-8
Martinez-Hernandez E, Amezcua-Allieri MA, Sadhukhan J, Anell JA (2018) Sugarcane bagasse valorization strategies for bioethanol and energy production. In: Sugarcane - Technology and Research. InTech
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20:295–299. https://doi.org/10.1016/j.copbio.2009.05.007
Debnath M, Pandey M, Sharma R, et al (2010) Biotechnological intervention of Agave sisalana: a unique fiber yielding plant with medicinal property. Academic Journals
Mancinoa A, Marannano G, Zuccarello B (2018) Implementation of eco-sustainable biocomposite materials reinforced by optimized agave fibers. In: Procedia Structural Integrity. Elsevier B.V., pp 526–538
Melkamu A, Kahsay MB, Tesfay AG (2019) Mechanical and water-absorption properties of sisal fiber (Agave sisalana)-reinforced polyester composite. J Nat Fibers 16:877–885. https://doi.org/10.1080/15440478.2018.1441088
Abebayehu SG, Engida AM (2021) Preparation of biocomposite material with superhydrophobic surface by reinforcing waste polypropylene with sisal (Agave sisalana) fibers. Int J Polym Sci 2021:1–15. https://doi.org/10.1155/2021/6642112
Sydenstricker THD, Mochnaz S, Amico SC (2003) Pull-out and other evaluations in sisal-reinforced polyester biocomposites. Polym Test 22:375–380. https://doi.org/10.1016/S0142-9418(02)00116-2
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291. https://doi.org/10.1007/s10295-003-0049-x
McMillan JD (1994) Conversion of hemicellulose hydrolyzates to ethanol. pp 411–437
Megiatto JD, Hoareau W, Gardrat C et al (2007) Sisal fibers: surface chemical modification using reagent obtained from a renewable source; characterization of hemicellulose and lignin as model study. J Agric Food Chem 55:8576–8584. https://doi.org/10.1021/jf071682d
Zwane PE, Ndlovu T, Mkhonta TT et al (2019) Effects of enzymatic treatment of sisal fibres on tensile strength and morphology. Sci African 6:e00136. https://doi.org/10.1016/j.sciaf.2019.e00136
González JTC, Dillon AJP, Pérez-Pérez AR et al (2015) Enzymatic surface modification of sisal fibers (Agave sisalana) by Penicillium echinulatum cellulases. Fibers Polym 16:2112–2120. https://doi.org/10.1007/s12221-015-4705-3
Mshandete A, Björnsson L, Kivaisi AK et al (2005) Enhancement of anaerobic batch digestion of sisal pulp waste by mesophilic aerobic pre-treatment. Water Res 39:1569–1575. https://doi.org/10.1016/j.watres.2004.11.037
Freier D, Mothershed CP, Wiegel J (1988) Characterization of Clostridium thermocellum JW20. Appl Environ Microbiol 54:204–211
Leis B, Held C, Bergkemper F et al (2017) Comparative characterization of all cellulosomal cellulases from Clostridium thermocellum reveals high diversity in endoglucanase product formation essential for complex activity. Biotechnol Biofuels 10:1–16. https://doi.org/10.1186/s13068-017-0928-4
Bayer EA, Morag E, Lamed R (1994) The cellulosome - a treasure-trove for biotechnology. Trends Biotechnol 12:379–386. https://doi.org/10.1016/0167-7799(94)90039-6
Fontes CMGA, Gilbert HJ (2010) Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu Rev Biochem 79:655–681. https://doi.org/10.1146/annurev-biochem-091208-085603
Fernandes AC, Fontes CMGA, Gilbert HJ, et al (1999) Homologous xylanases from Clostridium thermocellum: evidence for bi-functional activity, synergism between xylanase catalytic modules and the presence of xylan-binding domains in enzyme complexes
S Heinze M Mechelke P Kornberger et al 2017 Identification of endoxylanase XynE from Clostridium thermocellum as the first xylanase of glycoside hydrolase family GH141 OPEN Sci Rep https://doi.org/10.1038/s41598-017-11598-y
Khan MIM, Sajjad M, Sadaf S et al (2013) The nature of the carbohydrate binding module determines the catalytic efficiency of xylanase Z of Clostridium thermocellum. J Biotechnol 168:403–408. https://doi.org/10.1016/J.JBIOTEC.2013.09.010
Untergasser A, Cutcutache I, Koressaar T et al (2012) Primer3-new capabilities and interfaces. Nucleic Acids Res 40:1–12. https://doi.org/10.1093/nar/gks596
Nielsen H (2017) Predicting secretory proteins with SignalP. Protein Funct Predict Methods Protoc 59–73
Bimboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523. https://doi.org/10.1093/nar/7.6.1513
PRV Hamann de MB L Silva, TC Gomes, EF Noronha 2021 Assembling mini-xylanosomes with Clostridium thermocellum XynA, and their properties in lignocellulose deconstruction Enzyme Microb Technol 150 109887 https://doi.org/10.1016/J.ENZMICTEC.2021.109887
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. https://doi.org/10.1016/j.pep.2005.01.016
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Wittig I, Braun HP, Schägger H (2006) Blue native PAGE. Nat Protoc 1:418–428. https://doi.org/10.1038/nprot.2006.62
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
PRV Hamann DL Serpa AS Barreto da Cunha et al 2015 Evaluation of plant cell wall degrading enzyme production by Clostridium thermocellum B8 in the presence of raw agricultural wastes Int Biodeterior Biodegrad 105 https://doi.org/10.1016/j.ibiod.2015.08.013
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Miller GL (1959) Use of dinitrosaiicyiic acid reagent for determination of reducing sugar. Anal Chem 3:426–428
C Silva de OG, Aquino EN, Ricart CAO et al 2015 GH11 xylanase from Emericella nidulans with low sensitivity to inhibition by ethanol and lignocellulose-derived phenolic compounds FEMS Microbiol Lett 362 1 8 https://doi.org/10.1093/femsle/fnv094
Ullah SF, Souza A, Hamann PRV et al (2019) Structural and functional characterisation of xylanase purified from Penicillium chrysogenum produced in response to raw agricultural waste. Int J Biol Macromol 127. https://doi.org/10.1016/j.ijbiomac.2019.01.057
Ximenes E, Kim Y, Mosier N et al (2011) Deactivation of cellulases by phenols. Enzyme Microb Technol 48:54–60. https://doi.org/10.1016/J.ENZMICTEC.2010.09.006
Boukari I, O’Donohue M, Rémond C, Chabbert B (2011) Probing a family GH11 endo-β-1,4-xylanase inhibition mechanism by phenolic compounds: role of functional phenolic groups. J Mol Catal B Enzym 72:130–138. https://doi.org/10.1016/j.molcatb.2011.05.010
Sacco M, Millet J, Aubert JP (1984) Cloning and expression in Saccharomyces cerevisiae of a cellulase gene from Clostridium thermocellum. Ann l’Institut Pasteur / Microbiol 135:485–488. https://doi.org/10.1016/S0769-2609(84)80088-5
Cheng YF, Yang CH, Liu WH (2005) Cloning and expression of Thermobifida xylanase gene in the methylotrophic yeast Pichia pastoris. Enzyme Microb Technol 37:541–546. https://doi.org/10.1016/j.enzmictec.2005.04.006
Zhang G-M, Hu Y, Zhuang Y-H, et al (2009) Molecular cloning and heterologous expression of an alkaline xylanase from Bacillus pumilus HBP8 in Pichia pastoris. 24 371 379 https://doi.org/10.1080/10242420600768771
Sajjad M, Khan MIM, Akbar NS et al (2010) Enhanced expression and activity yields of Clostridium thermocellum xylanases without non-catalytic domains. J Biotechnol 145:38–42. https://doi.org/10.1016/J.JBIOTEC.2009.10.013
Grepinet O, Chebrou M-C, Beguin P (1988) Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum
Suzuki H, Imaeda T, Kitagawa T, Kohda K (2012) Deglycosylation of cellulosomal enzyme enhances cellulosome assembly in Saccharomyces cerevisiae. J Biotechnol 157:64–70. https://doi.org/10.1016/j.jbiotec.2011.11.015
Verma AK, Goyal A (2016) A novel member of family 30 glycoside hydrolase subfamily 8 glucuronoxylan endo-β-1,4-xylanase (CtXynGH30) from Clostridium thermocellum orchestrates catalysis on arabinose decorated xylans. J Mol Catal B Enzym 129:6–14. https://doi.org/10.1016/J.MOLCATB.2016.04.001
de Camargo BR, Claassens NJ, Quirino BF et al (2018) Heterologous expression and characterization of a putative glycoside hydrolase family 43 arabinofuranosidase from Clostridium thermocellum B8. Enzyme Microb Technol 109:74–83. https://doi.org/10.1016/j.enzmictec.2017.09.014
Osiro KO, de Camargo BR, Satomi R et al (2017) Characterization of Clostridium thermocellum (B8) secretome and purified cellulosomes for lignocellulosic biomass degradation. Enzyme Microb Technol 97:43–54. https://doi.org/10.1016/j.enzmictec.2016.11.002
Stern J, Anbar M, Moraïs S et al (2014) Insights into enhanced thermostability of a cellulosomal enzyme. Carbohydr Res 389:78–84. https://doi.org/10.1016/j.carres.2014.01.014
Kapoor M, Kuhad RC (2007) Immobilization of xylanase from Bacillus pumilus strain MK001 and its application in production of xylo-oligosaccharides. Appl Biochem Biotechnol 142:125–138. https://doi.org/10.1007/s12010-007-0013-8
Duarte GC, Moreira LRS, Jaramillo PMD, Filho EXF (2012) Biomass-derived inhibitors of holocellulases Bioenergy Res 5:768–777. https://doi.org/10.1007/s12155-012-9182-6
Ladeira Ázar RIS, Morgan T, dos Santos ACF et al (2018) Deactivation and activation of lignocellulose degrading enzymes in the presence of laccase. Enzyme Microb Technol 109:25–30. https://doi.org/10.1016/j.enzmictec.2017.09.007
AV Monclaro GL Recalde FG Silva da et al 2019 Xylanase from Aspergillus tamarii shows different kinetic parameters and substrate specificity in the presence of ferulic acid Enzyme Microb Technol https://doi.org/10.1016/j.enzmictec.2018.09.009
Van Dongen FEM, Van Eylen D, Kabel MA (2011) Characterization of substituents in xylans from corn cobs and stover. Carbohydr Polym 86:722–731. https://doi.org/10.1016/J.CARBPOL.2011.05.007
MP Pinheiro A. G. Reis R, Dupree P, Ward RJ, 2021 Plant cell wall architecture guided design of CBM3-GH11 chimeras with enhanced xylanase activity using a tandem repeat left-handed β-3-prism scaffold Comput Struct Biotechnol J https://doi.org/10.1016/j.csbj.2021.01.011
Hervé C, Rogowski A, Blake AW et al (2010) Carbohydrate-binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc Natl Acad Sci U S A 107:15293–15298. https://doi.org/10.1073/pnas.1005732107
Hyeon JE, Jeon SD, Han SO (2013) Cellulosome-based, Clostridium-derived multi-functional enzyme complexes for advanced biotechnology tool development: advances and applications. Biotechnol Adv 31:936–944. https://doi.org/10.1016/j.biotechadv.2013.03.009
Acknowledgements
Pedro R.V Hamann is a recipient of CAPES doctoral scholarship, Tainah C. Gomes, and Luísa de M.B Silva are recipients of CAPES master degree scholarship. Eliane F. Noronha is a research fellow of CNPq. This research was supported by grants from the University of Brasília and FAP-DF.
Author information
Authors and Affiliations
Contributions
Pedro R.V Hamann wrote the manuscript and conceived experiments; Tainah C. Gomes conceived experiments; Luísa de M.B Silva conceived experiments; Eliane F. Noronha revised the manuscript, conceived experiments, and was responsible for funding acquisition.
Corresponding author
Ethics declarations
Ethical statement
The manuscript was read and approved by all authors; the authors would like to declare that there is no conflict of interest, and credit has appropriately been given to everyone who contributed to the present study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Hydrolysis of sisal fibers using mini-xylanosomes generates three times more reducing sugar than non-complexed xylanase.
• Cellulose immobilized mini-xylanosomes could be subject to 72 h of reuse cycles at 50 and 60 °C.
• At 70 °C, xylanases assembled into mini-xylanosomes display better thermal stability than non-complexed.
• Complexing the xylanase into mini-xylanosomes does not change the hydrolysis yield of soluble oat spelt xylan.
Rights and permissions
About this article
Cite this article
Hamann, P.R.V., Gomes, T.C., Silva, L.d.M.B. et al. Using mini-xylanosomes as a biotechnological tool for sisal fiber deconstruction and enzyme immobilization. Biomass Conv. Bioref. 13, 12143–12155 (2023). https://doi.org/10.1007/s13399-021-02137-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02137-x