Abstract
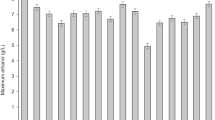
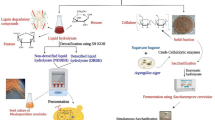
Biofuel derived from lignocellulosic biomasses recently emerged as one of the most suitable environmental friendly substrates that cannot only lead the renewable energy, but also minimizing the emission of greenhouse gases. Lignocellulosic materials are considered to be the major components as of yet unexploited source of for bioethanol production. The main aim of the current works was to discover the optimal bioethanol production from a local wild plants biomass Quercus aegilops, which are very common in Kurdistan region, Iraq. Pretreatment with different sulfuric acid concentrations at varied reaction times and temperatures was used. Three-step conversion process, separate hydrolysis, and fermentation (SHF) were used to evaluate the conversion of both cellulose and hemicellulose to monomeric sugars, and ethanol production. The results showed that the maximum yields of glucose and xylose were 5.7 g L−1 and 21.1 g L−1, respectively, when the biomass pretreated with 15% total solid, 2% acid concentrations at 120 °C for 120 min. During 48 h of enzymatic hydrolysis, the maximum glucose and xylose concentrations were 5.97 g L−1 and 17.53 g L−1, respectively, showing a conversion rate of 21.8% and 80.1% for the corresponding sugars. The highest ethanol yield of 6.01 g L−1 at 96 h fermentation with the productivity of 0.049 g L−1 h−1 was obtained when fermentation carried out with Pichia Stipitis yeast strain, while the maximum ethanol concentration and ethanol yield the productivity of 6.93 g L−1 and of 0.242 g L−1 h−1, respectively, were obtained during 24 h of fermentation by Kluyveromyces marxianus yeast strain. From the outcomes, it was obvious that K. marxianus was the favored yeast strain to be used for the generation of bioethanol from leaves of Q. aegilops, and Q. aegilops has potential to be exploited as a native, abundant raw material for the production of ethanol.




Similar content being viewed by others
References
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101(13):4744–4753
Outlook SAE (2015) World energy outlook special report. International Energy Agency 135
Njoku SI (2012) Optimization of the production of cellulosic biofuels: Ph. Aalborg University, Copenhagen, Denmark, D. Thesis
Zhou A, Thomson E (2009) The development of biofuels in Asia. Appl Energy 86:S11–S20
Yadav KS, Naseeruddin S, Prashanthi GS, Sateesh L, Rao LV (2011) Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol 102(11):6473–6478
Gabhane J, William SP, Gadhe A, Rath R, Vaidya AN, Wate S (2014) Pretreatment of banana agricultural waste for bio-ethanol production: individual and interactive effects of acid and alkali pretreatments with autoclaving, microwave heating and ultrasonication. Waste Manag 34(2):498–503
Ghafour NH, Baban MMR (2010) Primary metabolites and energy of oak fruits (Quercus spp.) in Khamza Mountain Oak Forest in Sulaimani/Iraqi Kurdistan Region. Journal of Chemistry and Chemical Engineering 4(6):15–21
Devendra LP, Pandey A (2016) Hydrotropic pretreatment on rice straw for bioethanol production. Renew Energy 98:2–8
Muhamad SG, Esmail LS, Hasan SH (2016) Kinetic studies of bioethanol production from wheat straw. ZANCO Journal of Pure and Applied Sciences 28(3):97–103
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35(2):489–511
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sust Energ Rev 32:713–728
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58
Dahnum D, Tasum SO, Triwahyuni E, Nurdin M, Abimanyu H (2015) Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Procedia 68:107–116
Azhar SHM, Abdulla R, Jambo SA, Marbawi H, Gansau JA, Faik AAM et al (2017) Yeasts in sustainable bioethanol production: a review. Biochemistry and Biophysics Reports 10:52–61
Tu Y, Wang L, Xia T, Sun D, Zhou S, Wang Y et al (2017) Mild chemical pretreatments are sufficient for complete saccharification of steam-exploded residues and high ethanol production in desirable wheat accessions. Bioresour Technol 243:319–326
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agriculture and Natural Resources 51(6):512–519
Ferreira JP, Miranda I, Sousa VB, Pereira H (2018) Chemical composition of barks from Quercus faginea trees and characterization of their lipophilic and polar extracts. PLoS One 13(5):e0197135
Arukwe U, Amadi B, Duru M, Agomuo E, Adindu E, Odika P et al (2012) Chemical composition of Persea americana leaf, fruit and seed. IJRRAS. 11(2):346–349
Tambe S (2012) Determination of ash values of some medicinal plant of Marathwada region in Maharashtra. Int J Pharm Res Biosci 1:337–346
Daud Z, Hatta MZM, Kassim ASM, Aripin AM (2014) Analysis of the chemical compositions and fiber morphology of pineapple (Ananas comosus) leaves in Malaysia. J Appl Sci 14(12):1355–1358
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40(12):3693–3700
Agu C, Njoku O, Chilaka F, Okorie S, Agbiogwu D (2012) Physico-chemical characterization of lignocellulosic fibre from Ampelocissus cavicaulis. Int J Basic & Appl Sci IJBAS-IJENS 12(3):68–77
Baber O, Slot M, Celis G, Kitajima K (2014) Diel patterns of leaf carbohydrate concentrations differ between seedlings and mature trees of two sympatric oak species. Botany 92(7):535–540
Khalil HSA, Alwani MS, Omar AKM (2006) Chemical composition, anatomy, lignin distribution, and cell wall structure of Malaysian plant waste fibers. BioResources 1(2):220–232
Raud M, Tutt M, Olt J, Kikas T (2015) Effect of lignin content of lignocellulosic material on hydrolysis efficiency. Agron Res 13(2):405–412
Lavarack B, Griffin G, Rodman D (2000) Measured kinetics of the acid-catalysed hydrolysis of sugar cane bagasse to produce xylose. Catal Today 63(2–4):257–265
Lu Y, Mosier NS (2007) Biomimetic catalysis for hemicellulose hydrolysis in corn Stover. Biotechnol Prog 23(1):116–123
Adsul M, Ghule J, Shaikh H, Singh R, Bastawde K, Gokhale D et al (2005) Enzymatic hydrolysis of delignified bagasse polysaccharides. Carbohydr Polym 62(1):6–10
Chen H. Chemical composition and structure of natural lignocellulose. Biotechnology of lignocellulose: Springer; 2014. p. 25–71
Lee J-W, Jeffries TW (2011) Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour Technol 102(10):5884–5890
Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources. 2(3):472–499
Soares I, Mendes K, Benachour M, Abreu C (2017) Evaluation of the effects of operational parameters in the pretreatment of sugarcane bagasse with diluted sulfuric acid using analysis of variance. Chem Eng Commun 204(12):1369–1390
El-Naggar N, Deraz S, Khalil A (2014) Bioethanol production from lignocellulosic feedstocks based on enzymatic hydrolysis: current status and recent developments. Biotechnology 13(1):1–21
Madadi M, Tu Y, Abbas A (2017) Recent status on enzymatic saccharification of lignocellulosic biomass for bioethanol production. Electron J Biol 13(2):135–143
Wyman CE, Spindler DD, Grohmann K (1992) Simultaneous saccharification and fermentation of several lignocellulosic feedstocks to fuel ethanol. Biomass Bioenergy 3(5):301–307
Chung Y-C, Bakalinsky A, Penner MH (2005) Enzymatic saccharification and fermentation of xylose-optimized dilute acid-treated lignocellulosics. Appl Biochem Biotechnol 124(1–3):947–961
Theerarattananoon K, Wu X, Staggenborg S, Propheter J, Madl R, Wang D (2010) Evaluation and characterization of sorghum biomass as feedstock for sugar production. Trans ASABE 53(2):509–525
Yoshida M, Liu Y, Uchida S, Kawarada K, Ukagami Y, Ichinose H et al (2008) Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci Biotechnol Biochem 72(3):805–810
de Souza AP, Grandis A, Leite DC, Buckeridge MS (2014) Sugarcane as a bioenergy source: history, performance, and perspectives for second-generation bioethanol. BioEnergy Research 7(1):24–35
Hindi S (2017) Differentiation and synonyms standardization of amorphous and crystalline cellulosic products. Nanosci Nanotechnol 4(3):73–85
Ranatunga TD, Jervis J, Helm RF, McMillan JD, Wooley RJ (2000) The effect of overliming on the toxicity of dilute acid pretreated lignocellulosics: the role of inorganics, uronic acids and ether-soluble organics. Enzym Microb Technol 27(3–5):240–247
Zhang Y, Xia C, Lu M, Tu M (2018) Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnology for biofuels 11(1):178
Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO (2001) Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Prog 17(2):287–293
Chandel AK, Chan E, Rudravaram R, Narasu ML, Rao LV, Ravindra P (2007) Economics and environmental impact of bioethanol production technologies: an appraisal. Biotechnology and Molecular Biology Review 2(1):14–32
Chandel AK, da Silva SS, Singh OV. Detoxification of lignocellulosic hydrolysates for improved bioethanol production. Biofuel production-recent developments and prospects: IntechOpen; 2011
Myat L, Ryu G (2016) Pretreatments and factors affecting saccharification and fermentation for lignocellulosic ethanol production. Cellulose Chem Technol 50:177–188
Acknowledgments
The authors thank Biosystems and Agricultural Engineering Department, Michigan State University, USA, for giving me the opportunity to work in their labs. We would like also to thank Michigan AgBioResearch for funding this work through faculty salaries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tahir, B., Mezori, H.A. Bioethanol production from Quercus aegilops using Pichia stipitis and Kluyveromyces marxianus. Biomass Conv. Bioref. 12, 3631–3640 (2022). https://doi.org/10.1007/s13399-020-00704-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00704-2