Abstract
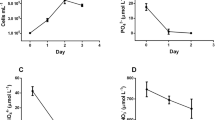
Cultivation conditions especially agitation and aeration have critical roles on Crypthecodinium cohnii growth and docosahexaenoic acid (DHA) formation. In this study, for the first time, different air bubble sizes were considered, in a 5-L bubble column bioreactor; and the effect of bubble size on growth and on DHA production by dinoflagellate microalgae C. cohnii was investigated. The cultivation was carried out under 0.5 vvm aeration flow rate, 28 °C, and pH ± 7. The highest yield of DHA (1.4 g DHA L−1) was achieved for 0.36-cm air bubble diameter; the corresponding biomass amount was 17.6 g. The suitable conditions during the logarithmic stage of growth were provided for 0.36-cm air bubble diameter, while oxygen limitation was observed in the culture medium afterwards. The concentration of the microalgal DHA was increased in oxygen limitation conditions. Our results indicate that the air bubble diameter is the most influencing factor on DHA production in a bubble column bioreactor. Current research indicates that optimum bubble size in bubble column bioreactor can lead to optimal conditions for growth and DHA production like two-stage oxygen supply feeding strategy.





Similar content being viewed by others
References
Barbosa MJ, Albrecht M, Wijffels RH (2003) Hydrodynamic stress and lethal events in sparged microalgae cultures. Biotechnol Bioeng 83:112–120
Anderson BM, Ma DW (2009) Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis 8:33
Cheng G, Fu C, Wang H, Adoligbe C, Wei S, Li S, Jiang B, Wang H, Zan L (2015) Production of transgenic beef cattle rich in n-3 PUFAs by somatic cell nuclear transfer. Biotechnol Lett 37:1565–1571
Ganuza E, Anderson A, Ratledge C (2008) High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol Lett 30:1559–1564
Lian M, Huang H, Ren L, Ji X, Zhu J, Jin L (2010) Increase of docosahexaenoic acid production by Schizochytrium sp. through mutagenesis and enzyme assay. Appl Biochem Biotechnol 162:935–941
Liu J, Sommerfeld M, Hu Q (2013) Screening and characterization of Isochrysis strains and optimization of culture conditions for docosahexaenoic acid production. Appl Microbiol Biotechnol 97:4785–4798
Sprague M, Betancor M, Tocher DR (2017) Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol Lett 39:1599–1609
Rumiani LA, Jalili H, Amrane A (2018) Enhanced docosahexaenoic acid production by Crypthecodinium cohnii under combined stress in two-stage cultivation with date syrup based medium. Algal Res 34:75–81
Song X, Tan Y, Liu Y, Zhang J, Liu G, Feng Y, Cui Q (2013) Different impacts of short-chain fatty acids on saturated and polyunsaturated fatty acid biosynthesis in Aurantiochytrium sp. SD116. J Agric Food Chem 61:9876–9881
Kyle D (1996) Production and use of a single cell oil which is highly enriched in docosahexaenoic acid. Lipid Technol 8:107–110 the text
De Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Sijtsma L, Anderson AJ, Ratledge C (2010) Alternative carbon sources for heterotrophic production of docosahexaenoic acid by the marine alga Crypthecodinium cohnii. In: Single cell oils, 2nd edn. Elsevier, Amsterdam, pp 131–149
Ratledge C (2010) Single cell oils for the 21st century. In: Single cell oils, 2nd edn. Elsevier, Amsterdam, pp 3–26
Beach D, Holz G Jr (1973) Environmental influences on the docosahexaenoate content of the triacylglycerols and phosphatidylcholine of a heterotrophic, marine dinoflagellate, Crypthecodinium cohnii. Biochim Biophys Acta Lipids Lipid Metab 316:56–65
Wang C, Lan CQ (2018) Effects of shear stress on microalgae–a review. Biotechnol Adv 36:986–1002
Pollingher U, Zemel E (1981) In situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma westii (Lemm.) Lefevre. Br Phycol J 16:281–287
Hu W, Gladue R, Hansen J, Wojnar C, Chalmers JJ (2007) The sensitivity of the dinoflagellate Crypthecodinium cohnii to transient hydrodynamic forces and cell-bubble interactions. Biotechnol Prog 23:1355–1362
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30:1525–1536
Hillig F, Annemüller S, Chmielewska M, Pilarek M, Junne S, Neubauer P (2013a) Bioprocess development in single-use systems for heterotrophic marine microalgae. Chem Ing Tech 85:153–161
Tuttle R, Loeblich A III (1975) An optimal growth medium for the dinoflagellate Crypthecodinium cohnii. Phycologia 14:1–8
Galleron C (1976) Synchronization of the marine dinoflagellate Amphidinium carteri in dense cultures. J Phycol 12:69–73
Gomes MD, Moiseyenko RP, Baum A, Jørgensen TM, Woodley JM (2019) Use of image analysis to understand enzyme stability in an aerated stirred reactor. Biotechnol Prog
Jung S-K (2019) A review of image analysis in biochemical engineering. Biotechnol Bioprocess Eng 24:65–75
de Swaaf ME, de Rijk TC, Eggink G, Sijtsma L (1999) Optimisation of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J Biotechnol 70:185–192
Ribeiro C Jr, Lage P (2004) Experimental study on bubble size distributions in a direct-contact evaporator. Braz J Chem Eng 21:69–81
Chen S, Timmons MB, Aneshansley DJ, Bisogni JJ Jr (1992) Bubble size distribution in a bubble column applied to aquaculture systems. Aquac Eng 11:267–280
Jiang Y, Chen F, Liang S-Z (1999) Production potential of docosahexaenoic acid by the heterotrophic marine dinoflagellate Crypthecodinium cohnii. Process Biochem 34:633–637
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161
Bandyopadhyay B, Humphrey A, Taguchi H (1967) Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol Bioeng 9:533–544
Chang G, Wu J, Jiang C, Tian G, Wu Q, Chang M, Wang X (2014) The relationship of oxygen uptake rate and kLa with rheological properties in high cell density cultivation of docosahexaenoic acid by Schizochytrium sp. S31. Bioresour Technol 152:234–240
Hu W, Gladue R, Hansen J, Wojnar C, Chalmers JJ (2010) Growth inhibition of dinoflagellate algae in shake flasks: not due to shear this time! Biotechnol Prog 26:79–87
Bi Z-Q, Ren L-J, Hu X-C, Sun X-M, Zhu S-Y, Ji X-J, Huang H (2018) Transcriptome and gene expression analysis of docosahexaenoic acid producer Schizochytrium sp. under different oxygen supply conditions. Biotechnol Biofuels 11:249
López-Rosales L, García-Camacho F, Sánchez-Mirón A, Contreras-Gómez A, Molina-Grima E (2015) An optimisation approach for culturing shear-sensitive dinoflagellate microalgae in bench-scale bubble column photobioreactors. Bioresour Technol 197:375–382
Ju J-H et al (2019) Boosting productivity of heterotrophic microalgae by efficient control of the oxygen transfer coefficient using a microbubble sparger. Algal research:101474
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K et al (2001) Production of polyunsaturated fatty acids by polyketidesynthases in both prokaryotes and eukaryotes. Science 293:290–293
Heathman TR, Nienow AW, Rafiq QA, Coopman K, Kara B, Hewitt CJ (2018) Agitation and aeration of stirred-bioreactors for the microcarrier culture of human mesenchymal stem cells and potential implications for large-scale bioprocess development. Biochem Eng J 136:9–17
Costariol E, Rotondi M, Amini A, Hewitt CJ, Nienow AW, Heathman TRJ, Micheletti M, Rafiq QA (2019) Establishing the scalable manufacture of primary human T-cells in an automated stirred-tank bioreactor. Biotechnol Bioeng
Chen C-Y, Yang Y-T (2018) Combining engineering strategies and fermentation technology to enhance docosahexaenoic acid (DHA) production from an indigenous Thraustochytrium sp. BM2 strain. Biochem Eng J 133:179–185
Hillig F, Porscha N, Junne S, Neubauer P (2014) Growth and docosahexaenoic acid production performance of the heterotrophic marine microalgae Crypthecodinium cohnii in the wave-mixed single-use reactor CELL-tainer. Eng Life Sci 14:254–263
Yeung P, Wong J (2003) Inhibition of cell proliferation by mechanical agitation involves transient cell cycle arrest at G 1 phase in dinoflagellates. Protoplasma 220:173–178
Hillig F, Pilarek M, Junne S, Neubauer P (2013b) Cultivation of marine microorganisms in single-use systems. In: Disposable bioreactors II. Springer, Berlin, pp 179–206
Acknowledgments
The authors would like to thank the staff of life science engineering and Micro-Electro-Mechanical Systems Laboratory for their assistance.
Author information
Authors and Affiliations
Contributions
The work was conceived and designed by Dr. H. J. The performance of experiments was done by N.H. The preparation of the manuscript was done by S.A. and N.H. Final revision of the manuscript was done by Dr. H. J. and Prof. A.A.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 247 kb)
Rights and permissions
About this article
Cite this article
Hoseinkhani, N., Jalili, H., Ansari, S. et al. Impact of bubble size on docosahexaenoic acid production by Crypthecodinium cohnii in bubble column bioreactor. Biomass Conv. Bioref. 11, 1137–1144 (2021). https://doi.org/10.1007/s13399-019-00510-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00510-5