Abstract
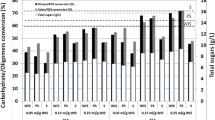
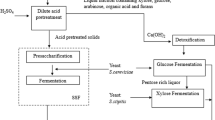
Lignocellulo-starch biomass (LCSB) comprising roots and vegetable processing wastes has high starch besides cellulose and hemicelluloses and warrants different pretreatment and saccharification approaches. The fermentable sugar yield from steam/dilute sulphuric acid (DSA)-pretreated biomass during saccharification with binary [cellulase + amylolytic enzyme (Stargen)] or triple (cellulase + xylanase + Stargen) enzyme cocktails was compared. The factors such as pH (5.0), temperature (50 °C) and enzyme dosage (16 FPU/g cellulose) for cellulase (Ecozyme RT80) action were optimized using response surface methodology. As pretreated liquor is rich in sugars, whole slurry saccharification was needed for LCSBs and saccharification efficiency (120 h) was significantly higher for steam-pretreated biomass with all application modes. Preferential hydrolysis of starch in steam-pretreated biomass by Stargen followed by cellulolysis was advantageous than the application sequence with cellulase followed by Stargen. Triple-enzyme-based saccharification of steam-pretreated biomass significantly enhanced the overall conversion efficiency (OCE; 85–98%) compared to only 28–49% in the native untreated biomass, while lower OCE was observed in the case of DSA-pretreated and saccharified biomass. Supplementation with both xylanase and Stargen pronouncedly enhanced the OCE for steam-pretreated biomass with only insignificant difference between the exposure periods, indicating the obligatory need for both enzymes for optimal saccharification of LCSBs.



Similar content being viewed by others
References
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Ener 37:19–28
Fuglestvedt J, Berntsen T, Myhre G, Rypdal K, Skeie RB (2008) Climate forcing from the transport sectors. Proc Natl Acad Sci U S A 105:454–458
Oghgren KH, Hahn B, Zacchi G (2006) Simultaneous saccharification and co- fermentation of glucose and xylose in steam pretreated corn stover at high fiber content with S. cerevisiae. J Biotech 126:488–496
Wyman CE (1999) Biomass ethanol: technical progress, opportunities and commercial challenges. Annu Rev Energ Environ 24:189–226
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 325:804–807
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low cost cellulosic ethanol. Biofuels Bioprod Bioref 2:26–40
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100:10–18
Taherzadeh MJ, Karimi K (2007) Enzyme-based hydrolysis process for ethanol production from lignocellulosic materials: a review. Bioresources 2(4):707–738
Leu SY, Zhu JY (2013) Substrate-related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. Bioener Res 6. doi:10.1007/s12155-012 9286-1
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Bioref 6(4):465–482
Yang B, Wyman CE (2004) Effect of xylan and lignin removal by batch and flow through pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol Bioeng 86(1):88–95
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:1–13
Zhang C, Zhuang XX, Wang ZJ, Matt F, St. John F, Zhu JY (2013) Xylanase supplementation on enzymatic saccharification of dilute acid pretreated poplars at different severities. Cellulose 20:1937–1946
Moxley G, Gaspar AR, Higgins D, Xu H (2012) Structural changes of corn stover lignin during acid pretreatment. J Indus Microbiol Biotechnol 39(9):1289–1299
Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR (2008) Synergistic enhancement of cellobiohydrolase performance on pretreated corn stover by addition of xylanase and esterase activities. Bioresour Technol 99:4997–5005
Murashima K, Kosugi A, Doi RH (2003) Synergistic effects of cellulosomal xylanase and cellulases from Clostridium cellulovorans on plant cell wall degradation. J Bacteriol 185(5):1518–1524
Keshwani DR, Cheng JJ (2009) Switchgrass for bioethanol and other value-added applications: a review. Bioresour Technol 100:137–145
Holtzapple MT, Ripley EP, Nikolaou M (1994) Saccharification, fermentation, and protein recovery from low-temperature AFEX-treated coastal Bermuda grass. Biotechnol Bioeng 44:1122–1131
Kim Y, Mosier NS, Ladisch MR (2009) Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol Prog 25:340–348
Zhu S, Wu Y, Yu Z, Wang C, Yu F et al (2006) Comparison of three microwave/chemical pretreatment processes for enzymatic hydrolysis of rice straw. Biosyst Eng 93:279–283
Xu J, Chen H, Kádár Z, Thomsen AB, Schmidt JE, Peng H (2011) Optimization of microwave pretreatment on wheat straw for ethanol production. Biomass Bioener 35:3859–3864
Benjamin Y, Cheng H, Görgens JF (2014) Optimization of dilute sulphuric acid pretreatment to maximize combined sugar yield form sugarcane bagasse for ethanol production. Appl Biochem Biotechnol 172:610–630
Mithra MG, Padmaja G (2016 a) Compositional profile and ultrastructure of steam and dilute sulfuric acid pretreated root and vegetable processing residues. Curr Biotechnol 7. doi:10.2174/2211550105666160916124120
Mithra MG, Padmaja G (2016 b) Lime pretreatment associated compositional and ultrastructural changes in selected root and vegetable processing residues. Amer J Biomass Bioener (In press).
Mithra MG, Padmaja G (2016 c) Comparative alterations in the compositional profile of selected root and vegetable peels subjected to three pretreatments for enhanced saccharification. Ind J Biotechnol (In press).
Lin L, Yan R, Liu Y, Jiang W (2010) In-depth investigation of enzymatic hydrolysis of biomass wastes based on three major components: cellulose, hemicelluloses and lignin. Bioresour Technol 101:8217–8223
Saha BC, Cotta MA (2009) Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. New Biotechnol 27:10–16
Divya Nair MP, Padmaja G, Moorthy SN (2011) Biodegradation of cassava starch factory residue using a combination of cellulases, xylanases and hemicellulases. Biomass Bioener 35:1211–1218
Saha BC, Bothast RJ (1999) Pretreatment and enzymatic saccharification of corn fiber. Appl Biochem Biotechnol 76:65–77
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Tomaz T, Roche A (2002) Hydrophobic interaction, chromatography of Trichoderma reesei cellulase on polypropylene glycol-sepharose. Separation Sci Technol 37:1–11
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem 153:375–380
Anon (2009) STARGEN™ 002: Granular starch hydrolyzing enzyme for ethanol production. Product information published by Genencor International, a Division of Danisco, Danisco US Inc., Available from: http://www.genencor.com. Accessed December 22, 2014.
AOAC (1995) Official Methods of Analysis, Sixteenth ed., Washington, DC: Association of Official Analytical Chemists.
Box G, Behnken D (1960) Some new three level designs for the study of quantitative variables. Techometrics 2:455–475
SAS (2010) Cary NC. USA: SAS Institute Inc.
Dien SS, Ximenes EA, O’Brien PJ, Moniruzzaman M, Li X-L, Balan V, Dale B, Cotta MA (2008) Enzyme characterization for hydrolysis of AFEX and liquid hot-water pretreated distillers’ grains and their conversion to ethanol. Bioresour Technol 99:5216–5225
Saha BC, Cotta MA (2010) Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. New Biotechnol 28:10–16
Bothast RJ, Saha BC (1997) Ethanol production from agricultural biomass substrates. Adv Appl Microbiol 44:261–286
Qing Q, Yang B, Wyman CE (2010) Xylo-oligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101:9624–9630
Mosier M, Wyman CE, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Lamptey J, Robinson CW, Moo-Young M (1985) Enhanced enzymatic hydrolysis of lignocellulosic biomass pretreated by low-pressure steam autohydrolysis. Biotechnol Letters 7:531–536
Mok WSL, Antal MJ (1992) Uncatalyzed solvolysis of whole biomass hemicelluloses by hot compressed liquid water. Ind Eng Chem Res 31:1157–1161
Bisaria VS, Ghose TK (1981) Biodegradation of cellulosic materials: substrate, microorganisms, enzymes and products. Enz Microb Technol 3:90–104
Tejirian A, Fu F (2011) Inhibition of enzymatic cellulolysis by phenolic compounds. Enz Microb Technol 48:239–247
Ximenes EA, Kim Y, Mosier NS, Dien BS, Ladisch MR (2010) Inhibition of cellulases by phenols. Enz Microb Technol 46:170–176
Mithra MG, Padmaja G (2016 d) Phenolic inhibitors of saccharification and fermentation in lignocellulo-starch prehydrolysates and comparative efficacy of detoxification treatments. J Biomass Biofuel 3:1–15. doi:10.11159/jbb.2016.001
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99:4201–4212
Pienkos PT, Zhang M (2009) Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 16:743–762
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
García-Aparicio MP, Ballesteros M, Manzanares P, Ballesteros I, González A, Negro MJ (2007) Xylanase contribution to the efficiency of cellulose enzymatic hydrolysis of barley straw. Appl Biochem Biotechnol 137–140:353–365
Yu P, Mckinon JJ, Maenz DD, Olkowski AA, Raez VJ, Christensen DA (2003) Enzymic release of reducing sugars from oat hulls by cellulase, as influenced by Aspergillus ferulic acid esterase and Trichoderma xylanase. J Agric Food Chem 51:218–223
Kumar R, Wyman CE (2009 a) Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol Prog 25:302–324
Qing Q, Wyman CE (2011) Supplementation with xylanase and ß-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol Biofuels 4(1):2–12
Xiao Z, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113–116:1115–1126
Kumar R, Wyman CE (2009 b) Effect of enzyme supplementation at moderate cellulase loadings on initial glucose and xylose release from corn stover solids pretreated by leading technologies. Biotechnol Bioeng 102:457–467
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour Technol 96:2026–2032
Acknowledgements
The authors acknowledge with gratitude the financial support from the Kerala State Council for Science, Technology & Environment (Grant no. 853/2015/KSCSTE) and the facilities provided by the director, ICAR-CTCRI, for the study. Stargen™ 002 was received by courtesy from M/s Danisco US Inc., USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mithra, M., Sreekumar, J. & Padmaja, G. Binary- and triple-enzyme cocktails and their application mode affect fermentable sugar release from pretreated lignocellulo-starch biomass. Biomass Conv. Bioref. 8, 97–111 (2018). https://doi.org/10.1007/s13399-017-0237-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-017-0237-y