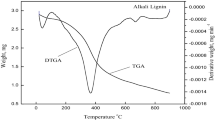
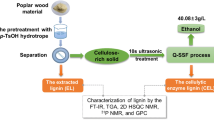
Abstract
The phenol-based antioxidants as free radical scavengers are expected to continue increasing as additives in industries. As an antioxidant, lignin has low oxidative stability in pure solid form, so stabilization is needed to produce lignin-based antioxidants. This research focuses on the valorization of alkaline lignin by hydrogenolysis using a Pt/C catalyst and formic acid as a hydrogen donor. Three treatment variables, namely the amount of formic acid (FA), the amount of ethanol (EtOH), and radiation time (T), were observed for their contribution to the three responses, namely the total phenol content (TPC), the degree of depolymerization (DD), and the IC50 value of DPPH as an antioxidants activity. This study found that the best results were obtained at operating conditions FA, EtOH, and T 5 mL, 100 mL, 3 min in sequence, producing TPC, DD, and IC50 worth 4792,055 mg/ L, 23.650%, and 35.860 mg/L, respectively. The results were optimized using the response surface methodology with the Design Expert 11 program with the optimized result being FA, EtOH, and T 10 mL, 100 mL, 3 min consecutively and resulted in TPC, DD, and IC50 of 4987.12 mg/L, 26.451%, and 33.865 mg/L, respectively. The results of this study indicate that OPFEB is a source of lignin that has the potential to produce phenolic compounds, which are sources of renewable fine chemicals based on biomass.





Similar content being viewed by others
Availability of Data and Materials
Availability of data and materials is not applicable.
References
Rahayu, D.; Karnaningroem, N.; Altway, A.; Slamet, A.: Utilization of oil palm empty fruit bunches biomass through slow pyrolysis process. IOP Conf. Ser. Earth Environ. Sci. 913(1), 012018 (2021). https://doi.org/10.1088/1755-1315/913/1/012018
Central statistics agency (BPS) (2021). Indonesian oil palm statistics 2021. [Online] Available: https://www.bps.go.id/publication/2022/11/30/254ee6bd32104c00437a4a61/statistik-kelapa-sawit-indonesia-2021.html
Dewanti, D.P.: Potensi Selulosa dari Limbah Tandan Kosong Kelapa Sawit untuk Bahan Baku Bioplastik Ramah Lingkungan Cellulose potential of empty fruit bunches waste as the raw material of bioplastics environmentally friendly. J. Teknol. Lingkungan (2018). https://doi.org/10.29122/jtl.v19i1.2644
Mohtar, S.S.; Busu, T.N.Z.T.M.; Noor, A.M.M.; Shaari, N.; Mat, H.: An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohyd. Polym. 166, 291–299 (2017). https://doi.org/10.1016/j.carbpol.2017.02.102
Gozan, M.; Panjaitan, J.R.; Tristantini, D.; Alamsyah, R.; Yoo, Y.J.: Evaluation of separate and simultaneous kinetic parameters for levulinic acid and furfural production from pretreated palm oil empty fruit bunches. Int. J. Chem. Eng. (2018). https://doi.org/10.1155/2018/1920180
Pizzi, A.: Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J. Adhes. Sci. Technol. 20(8), 829–846 (2006). https://doi.org/10.1163/156856106777638635
Hu, L.; Pan, H.; Zhou, Y.; Zhang, M.: Methods to improve lignin’s reactivity as a phenol substitute and as replacement for other phenolic compounds: a brief review. BioResources 6(3), 3515–3525 (2011)
Mankar, S., Chaudhari, A., Soni, I.: "Lignin in phenol-formaldehyde adhesives". Int. J. Knowl. Eng., ISSN, pp. 0976–5816, 2012
Rowell, R.M.: Handbook of wood chemistry and wood composites. CRC Press (2012). https://doi.org/10.1201/b12487
Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R.: Lignin-based copolymer adhesives for composite wood panels—a review. Int. J. Adhes. Adhes. 95, 102408 (2019). https://doi.org/10.1016/j.ijadhadh.2019.102408
Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U.: Characterization of the radical scavenging activity of lignins––natural antioxidants. Biores. Technol. 95(3), 309–317 (2004). https://doi.org/10.1016/j.biortech.2004.02.024
Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N.: Organosolv ethanol lignin from hybrid poplar as a radical scavenger: relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 54(16), 5806–5813 (2006). https://doi.org/10.1021/jf0605392
Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Sun, R.-C.; Jones, G.L.: Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind. Crops Prod. 56, 128–136 (2014). https://doi.org/10.1016/j.indcrop.2014.02.031
Chen, K.; Ye, D.; Gu, S.; Zhou, Y.: Thermal-oxidative effect of kraft lignin antioxidant in polypropylene: uncovering the key factor using correlation analysis model. Int. J. Biol. Macromol. 107, 478–485 (2018). https://doi.org/10.1016/j.ijbiomac.2017.09.016
Gosslink, R.; De Jong, E.; Guran, B.; Abacherli, A.: Co-ordination network for lignin-standardisation, production and applications adapted to market requirements. Ind. Crops Prod. 20, 121–129 (2004). https://doi.org/10.1016/j.indcrop.2004.04.015
Piccinino, D.; Capecchi, E.; Tomaino, E.; Gabellone, S.; Gigli, V.; Avitabile, D.; Saladino, R.: Nano-structured lignin as green antioxidant and UV shielding ingredient for sunscreen applications. Antioxidants 10(2), 274 (2021). https://doi.org/10.3390/antiox10020274
Harahap, A.F.P.; Gozan, M.: Utilization of lignin as natural antioxidant for biodiesel. AIP Conf. Proc. (2018). https://doi.org/10.1063/1.5064310
Harahap, A.F.; Rahman, A.A.; Sadrina, I.N.; Gozan, M.: Optimization of pretreatment conditions for microwave-assisted alkaline delignification of empty fruit bunch by response surface methodology. Optimization (2019). https://doi.org/10.14716/ijtech.v10i8.3431
Zhao, L.; Ouyang, X.; Ma, G.; Qian, Y.; Qiu, X.; Ruan, T.: Improving antioxidant activity of lignin by hydrogenolysis. Indus. Crops Prod. 1(125), 228–235 (2018). https://doi.org/10.1016/j.indcrop.2018.09.002
Brand-Williams, W.; Cuvelier, M.-E.; Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28(1), 25–30 (1995). https://doi.org/10.1016/S0023-6438(95)80008-5
Singleton, V. L., Orthofer, R., Lamuela-Raventós, R. M.: "[14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent". In: Methods in enzymology, vol. 299: Academic Press, 1999, pp. 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Khan, R.J.; Lau, C.Y.; Guan, J.; Lam, C.H.; Zhao, J.; Ji, Y.; Wang, H.; Xu, J.; Lee, D.J.; Leu, S.Y.: Recent advances of lignin valorization techniques toward sustainable aromatics and potential benchmarks to fossil refinery products. Biores. Technol. 346, 126419 (2022). https://doi.org/10.1016/j.biortech.2021.126419
Ye, K.; Liu, Y.; Wu, S.; Zhuang, J.: A review for lignin valorization: challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 172, 114008 (2021). https://doi.org/10.1016/j.indcrop.2021.114008
Milovanovic, J.; Rajic, N.; Romero, A.A.; Li, H.; Shih, K.; Tschentscher, R.; Luque, R.: Insights into the microwave-assisted mild deconstruction of lignin feedstocks using NiO-containing ZSM-5 zeolites. ACS Sustain. Chem. Eng. 4(8), 4305–4313 (2016)
Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H.: Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 114(3), 806–812 (2009). https://doi.org/10.1016/j.foodchem.2008.10.017
Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J.: Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 8(43), 24525–24533 (2018). https://doi.org/10.1039/C8RA02255K
Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J.: Lignins from agroindustrial by-products as natural ingredients for cosmetics: chemical structure and in vitro sunscreen and cytotoxic activities. Molecules 25(5), 1131 (2020). https://doi.org/10.3390/molecules25051131
Gordobil, O.; Oberemko, A.; Saulis, G.; Baublys, V.; Labidi, J.: In vitro cytotoxicity studies of industrial Eucalyptus kraft lignins on mouse hepatoma, melanoma and Chinese hamster ovary cells. Int. J. Biol. Macromol. 135, 353–361 (2019). https://doi.org/10.1016/j.ijbiomac.2019.05.111
de Menezes, N.I.; Avelino, F.; de Oliveira, D.R.; Souza, N.F.; Rosa, M.F.; Mazzetto, S.E.; Lomonaco, D.: Organic solvent fractionation of acetosolv palm oil lignin: the role of its structure on the antioxidant activity. Int. J. Biol. Macromol. 1(122), 1163–1172 (2019). https://doi.org/10.1016/j.ijbiomac.2018.09.066
Tavares, D.; Cavali, M.; Tanobe, V.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Filho, A.Z.; Soccol, C.R.; Woiciechowski, A.L.: Lignin from residual sawdust of Eucalyptus spp.—isolation, characterization, and evaluation of the antioxidant properties. Biomass 2(3), 195–208 (2022). https://doi.org/10.3390/biomass2030013
Li, Z.; Zhang, J.; Qin, L.; Ge, Y.: Enhancing antioxidant performance of lignin by enzymatic treatment with laccase. ACS Sustain. Chem. Eng. 6(2), 2591–2595 (2018). https://doi.org/10.1021/acssuschemeng.7b04070
Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł: Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 13(6), e0197044 (2018). https://doi.org/10.1371/journal.pone.0197044
Litwinienko, G.; Ingold, K.: Solvent effects on the rates and mechanisms of reaction of phenols with free radicals. Acc. Chem. Res. 40(3), 222–230 (2007). https://doi.org/10.1021/ar0682029
Craft, B.D.; Kerrihard, A.L.; Amarowicz, R.; Pegg, R.B.: Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 11(2), 148–173 (2012). https://doi.org/10.1111/j.1541-4337.2011.00173.x
Ansaloni, S.; Russo, N.; Pirone, R.: Wet air oxidation of industrial lignin case study: influence of the dissolution pretreatment and perovskite-type oxides. Waste Biomass Valoriz. 9, 2165–2179 (2018). https://doi.org/10.1007/s12649-017-9947-4
Shao, L.; Wang, C.; Liu, Y.; Wang, M.; Wang, L.; Xu, F.: Efficient depolymerization of lignin through microwave-assisted Ru/C catalyst cooperated with metal chloride in methanol/formic acid media. Front. Bioeng. Biotechnol. 16(10), 1082341 (2022). https://doi.org/10.3389/fbioe.2022.1082341
Dhar, P.; Vinu, R.: Microwave-assisted catalytic solvolysis of lignin to phenols: kinetics and product characterization. ACS Omega 3(11), 15076–15085 (2018). https://doi.org/10.1021/acsomega.8b01509
Nammalwar, B.; Bunce, R.A.; Berlin, K.D.; Bourne, C.R.; Bourne, P.C.; Barrow, E.W.; Barrow, W.W.: Approaches to iodinated derivatives of vanillin and isovanillin. Org. Prep. Proced. Int. 44(2), 146–152 (2012). https://doi.org/10.1080/00304948.2012.643700
Rodrigues Pinto, P.C.; Borges da Silva, E.A.; Rodrigues, A.E.: Lignin as source of fine chemicals: vanillin and syringaldehyde. Biomass Convers. Interface Biotechnol. Chem. Mater. Sci. 1, 1 (2012)
Baker, C.J.; Mock, N.M.; Whitaker, B.D.; Roberts, D.P.; Rice, C.P.; Deahl, K.L.; Averyanov, A.A.: Involvement of acetosyringone in plant—pathogen recognition. Biochem. Biophys. Res. Commun. 328(1), 130–136 (2005). https://doi.org/10.1016/j.bbrc.2004.12.153
Shi, Y.; Chai, L.; Tang, C.; Yang, Z.; Zheng, Y.; Chen, Y.; Jing, Q.: Biochemical investigation of kraft lignin degradation by Pandoraea sp. B-6 isolated from bamboo slips. Bioprocess Biosyst. Eng. 36, 1957–1965 (2013). https://doi.org/10.1007/s00449-013-0972-9
de Armas-Ricard, M.; Ruiz-Reyes, E.; Ramírez-Rodríguez, O.: Caffeates and caffeamides: synthetic methodologies and their antioxidant properties. Int. J. Med. Chem. (2019). https://doi.org/10.1155/2019/2592609
Chen, I.-S.; Lin, Y.C.; Tsai, I.L.; Teng, C.M.; Ko, F.N.; Ishikawa, T.; Ishii, H.: Coumarins and anti-platelet aggregation constituents from Zanthoxylum schinifolium. Phytochemistry 39(5), 1091–1097 (1995). https://doi.org/10.1016/0031-9422(95)00054-B
Bayati, S.; Yazdanparast, R.: Antioxidant and free radical scavenging potential of yakuchinone B derivatives in reduction of lipofuscin formation using H2O2-treated neuroblastoma cells. Iran. Biomed. J. 15(4), 134 (2011). https://doi.org/10.6091/ibj.1010.2012
Roy, M.; Chakraborty, S.; Siddiqi, M.; Bhattacharya, R.K.: Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac. J. Cancer Prev. 3(1), 61–67 (2002)
Razgonova, M.; Zakharenko, A.; Pikula, K.; Kim, E.; Chernyshev, V.; Ercisli, S.; Cravotto, G.; Golokhvast, K.: Rapid mass spectrometric study of a supercritical CO2-extract from woody liana Schisandra chinensis by HPLC-SPD-ESI-MS/MS. Molecules 25(11), 2689 (2020). https://doi.org/10.3390/molecules25112689
Kim, H.W.; Shin, J.H.; Lee, M.K.; Jang, G.H.; Lee, S.H.; Jang, H.H.; Jeong, S.T.; Kim, J.B.: Qualitative and quantitative analysis of dibenzocyclooctadiene lignans for the fruits of Korean. Korean J. Med. Crop Sci. 23(5), 385–394 (2015). https://doi.org/10.7783/KJMCS.2015.23.5.385
Zhu, G.; Ouyang, X.; Yang, Y.; Ruan, T.; Qiu, X.: Selective cleavage of aryl ether bonds in dimeric lignin model compounds. RSC Adv. 6(22), 17880–17887 (2016). https://doi.org/10.1039/C5RA26235F
Tripathi, M.; Bhatnagar, A.; Mubarak, N.M.; Sahu, J.N.; Ganesan, P.: RSM optimization of microwave pyrolysis parameters to produce OPS char with high yield and large BET surface area. Fuel 277, 118184 (2020). https://doi.org/10.1016/j.fuel.2020.118184
Cao, N.; Chen, Y.; Lu, K.; Wu, C.; Abudila, B.; Li, J.; Liu, C.L.; Dong, W.S.: Selective hydrogenolysis of 5-hydroxymethylfurfural to 2, 5-dimethylfuran with ethanol as a hydrogen donor over β-Mo 2 C embedded in carbon microspheres. Sustain. Energy Fuels 5(18), 4749–4757 (2021). https://doi.org/10.1039/D1SE00499A
He, Y.; Deng, L.; Lee, Y.; Li, K.; Lee, J.M.: A review on the critical role of H2 donor in the selective hydrogenation of 5-hydroxymethylfurfural. Chem. Sus. Chem. 15(13), e202200232 (2022). https://doi.org/10.1002/cssc.202200232
Ouyang, X.; Huang, X.; Zhu, Y.; Qiu, X.: Ethanol-enhanced liquefaction of lignin with formic acid as an in situ hydrogen donor. Energy Fuels 29(9), 5835–5840 (2015). https://doi.org/10.1021/acs.energyfuels.5b01127
Gupta, B.S.; Ako, J.E.: Application of guar gum as a flocculant aid in food processing and potable water treatment. Eur. Food Res. Technol. 221(6), 746–751 (2005). https://doi.org/10.1007/s00217-005-0056-4
Ramakrishna, G.; Susmita, M.: Application of response surface methodology for optimization of Cr (III) and Cr (VI) adsorption on commercial activated carbons. Res. J. Chem. Sci. 2231, 606X (2012)
Acknowledgements
The authors thank Talent Management of the National Research and Innovation Agency (BRIN) for supporting the first author in the Post-Doctoral Research Program. The authors also acknowledge the facilities and scientific and technical support from the National Research and Innovation Agency through e-Layanan Sains, Badan Riset dan Inovasi Nasional.
Funding
This research was supported by the National Research and Innovation Agency of Indonesia and Indonesia Endowment Fund for Education financial support through the research grant “Riset Inovasi untuk Indonesia Maju” (RIIM) 2022 (contract number: 97/IV/KS/11/2022). The authors also gratefully acknowledged the financial support for publication by the National Research and Innovation Agency financial support through the research grant “Rumah program Energi Terbarukan Organisasi Riset Energi dan Manufaktur” (RP ET OREM) 2023. (Contract number: 13/III.3/HK/2022).
Author information
Authors and Affiliations
Contributions
M.A Darmawan, M.Y.A Ramadhan, and M. Gozan are the main contributors who conceptualized, designed, and wrote this work. H. Hidayat and Muryanto supported obtaining the lignin extraction data from OPFEB. N. Amir and H. Heriyanto supported the production of phenolic compounds. H. Saputra and S.D.S Murti supported the interpretation and analysis of the data. T.S Utami supported for statistical analysis.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Ethical Approval
This article does not contain any studies with human or animal participants.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Darmawan, M.A., Ramadhan, M.Y.A., Saputra, H. et al. Production and Optimization of Hydroxy and Methyl Phenolic Compounds Through Microwave-Assisted Catalytic Hydrogenolysis from Lignin Valorization. Arab J Sci Eng 49, 8425–8441 (2024). https://doi.org/10.1007/s13369-024-08897-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-024-08897-8