Abstract
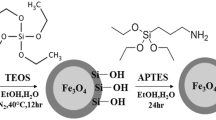
The cyclic oligosaccharide β-cyclodextrin (β-CD) can be widely employed for the preparation of controlled drug delivery systems. Hence, we synthesized a novel β-CD-based magnetic nanocarrier with superparamagnetic and molecular recognition properties for the controlled release of doxorubicin (DOX) drug. The magnetic nanocarrier was prepared by using a facile ultrasonic-assisted method and through surface reversible addition fragmentation chain transfer copolymerization of core–shell Fe3O4@SiO2 nanoparticles onto vinylated β-CD. The spherical amino-functionalized Fe3O4@SiO2 nanomaterials were synthesized easily by a co-condensation green reaction between Fe3O4 nanoparticles with tetraethyl orthosilicate and (3-Aminopropyl) trimethoxysilane. In the next age, the structure and composition of the as-prepared magnetic drug delivery nanocarriers were studied by FTIR, SEM, TEM, XRD, and VSM analysis methods. Then, the loading and releasing behaviors of DOX drug have been investigated in detail. The loading of DOX was proved by UV–Vis, SEM, TEM, and Brunauer–Emmett–Teller analysis techniques. The results also exhibited that the drug loading efficiency is found to be dependent on initial drug concentration, β-CD content and temperature. Moreover, the drug loading capacity of magnetic nanocarrier was compared with pure β-CD. Further, it was found that the in vitro release rate of DOX depends on the pH of the physiological solution. In addition, the drug release kinetics data were well fitted to the Higuchi model. Finally, the biological characterizations were tested by MTT assay, which approved the high performance of the magnetic nanocarriers in killing cancerous cells. Due to its unique and distinct advantageous, the synthesized magnetic nanocarriers can be utilized as a potential and promising biodegradable drug carrier for controlled and sustained release of various drugs.













Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Pechar, M.; Pola, R.; Studenovský, M.; Bláhová, M.; Grosmanová, E.; Dydowiczová, A.; Filipová, M.; Islam, R.; Gao, S.; Fang, J.; Etrych, T.: Polymer nanomedicines with enzymatically triggered activation: a comparative study of in vitro and in vivo anti-cancer efficacy related to the spacer structure. Nanomed. Nanotechnol. Biol. Med. 46, 102597–102608 (2022). https://doi.org/10.1016/j.nano.2022.102597
Kopeček, J.; Yang, J.: Polymer nanomedicines. Adv. Drug Deliv. Rev. 156, 40–64 (2020). https://doi.org/10.1016/j.addr.2020.07.020
Karthikeyan, L.; Sobhana, S.; Yasothamani, V.; Gowsalya, K.; Vivek, R.: Multifunctional theranostic nanomedicines for cancer treatment: recent progress and challenges. Biomed. Eng. Adv. 5, 100082–100098 (2023). https://doi.org/10.1016/j.bea.2023.100082
Lombardo, D.; Kiselev, M.A.; Caccamo, M.T.: Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 1–26 (2019). https://doi.org/10.1155/2019/3702518
Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C.: Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 17, 20–37 (2017). https://doi.org/10.1038/nrc.2016.108
Cheng, Z.; Li, M.; Dey, R.; Chen, Y.: Nanomaterials for cancer therapy: current progress and perspectives. J. Hematol. Oncol. 14, 85–91 (2021). https://doi.org/10.1186/s13045-021-01096-0
Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P.: Cancer nanomedicine: a review of recent success in drug delivery. Clin. Transl. Med. 6, 1–21 (2017). https://doi.org/10.1186/s40169-017-0175-0
Bahloul, B.; Castillo-Henríquez, L.; Jenhani, L.; Aroua, N.; Ftouh, M.; Kalboussi, N.; Vega-Baudrit, J.; Mignet, N.: Nanomedicine-based potential phyto-drug delivery systems for diabetes. J. Drug Deliv. Sci. Technol. 82, 104377–104383 (2023). https://doi.org/10.1016/j.jddst.2023.104377
Khot, V.M.; Salunkhe, A.B.; Pricl, S.; Bauer, J.; Thorat, N.D.; Townley, H.: Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Discov. Today 26, 724–739 (2021). https://doi.org/10.1016/j.drudis.2020.12.016
Li, J.; Burgess, D.J.: Nanomedicine-based drug delivery towards tumor biological and immunological microenvironment. Acta Pharm. Sin. B. 10, 2110–2124 (2020). https://doi.org/10.1016/j.apsb.2020.05.008
Qiu, Y.: Environment-sensitive hydrogels for drug delivery. Adv. Drug Del. Rev. 53, 321–339 (2011). https://doi.org/10.1016/s0169-409x(01)00203-4
Li, Z.; Du, X.; Cui, X.; Wang, Z.: Ultrasonic-assisted fabrication and release kinetics of two model redox-responsive magnetic microcapsules for hydrophobic drug delivery. Ultrason. Sonochem. 57, 223–232 (2019). https://doi.org/10.1016/j.ultsonch.2019.04.037
Perecin, C.J.; Sponchioni, M.; Auriemma, R.; Cerize, N.N.P.; Moscatelli, D.; Varanda, L.C.: Magnetite nanoparticles coated with biodegradable zwitterionic polymers as multifunctional nanocomposites for drug delivery and cancer treatment. ACS Appl. Nano Mater. 5, 16706–16719 (2022). https://doi.org/10.1021/acsanm.2c03712
Fang, Z.; Pan, S.; Gao, P.; Sheng, H.; Li, L.; Shi, L.; Zhang, Y.; Cai, X.: Stimuli-responsive charge-reversal nano drug delivery system: the promising targeted carriers for tumor therapy. Int. J. Pharm. 575, 118841–118849 (2020). https://doi.org/10.1016/j.ijpharm.2019.118841
Sun, T.; Jiang, C.: Stimuli-responsive drug delivery systems triggered by intracellular or subcellular microenvironments. Adv. Drug Deliv. Rev. 196, 114773–114781 (2023). https://doi.org/10.1016/j.addr.2023.114773
Alsehli, M.: Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: recent advances in drug delivery. Saudi Pharm. J. 28, 255–265 (2020). https://doi.org/10.1016/j.jsps.2020.01.004
Tian, B.; Liu, J.: Smart stimuli-responsive chitosan hydrogel for drug delivery: a review. Int. J. Biol. Macromol. 235, 123902–123909 (2023). https://doi.org/10.1016/j.ijbiomac.2023.123902
Jackson, T.C.; Obiakor, N.M.; Iheanyichukwu, I.N.; Ita, O.O.; Ucheokoro, A.S.: Biotechnology and nanotechnology drug delivery: a review. J. Pharm. Pharmacol. 9, 127–132 (2021). https://doi.org/10.17265/2328-2150/2021.04.001
Khan, I.; Khan, M.; Umar, M.N.; Oh, D.H.: Nanobiotechnology and its applications in drug delivery system: a review. IET Nanobiotechnol. 9, 396–400 (2015). https://doi.org/10.1049/iet-nbt.2014.0062
Crommelin, D.J.; Storm, G.; Jiskoot, W.; Stenekes, R.; Mastrobattista, E.; Hennink, W.E.: Nanotechnological approaches for the delivery of macromolecules. J. Control. Rel. 87, 81–88 (2003). https://doi.org/10.1016/s0168-3659(03)00014-2
Peppas, N.A.: Intelligent therapeutics: biomimetic systems and nanotechnology in drug delivery. Adv. Drug Deliv. Rev. 56, 1529–1531 (2004). https://doi.org/10.1016/j.addr.2004.07.001
Subbiah, R.; Veerapandian, M.; Yun, K.S.: Nanoparticles-functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 17, 4559–4577 (2010). https://doi.org/10.2174/092986710794183024
Ying, Z.; Chan, H.F.; Leong, K.W.: Advanced materials and processing for drug delivery: the past and the future. Adv. Drug Deliv. Rev. 65, 104–120 (2013). https://doi.org/10.1016/j.addr.2012.10.003
Li, C.; Wang, J.; Wang, Y.; Jin, Y.: Recent progress in drug delivery. Acta Pharm. Sin. B 9, 1145–1162 (2019). https://doi.org/10.1016/j.apsb.2019.08.003
Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M.: Nanocarriers-mediated drug delivery systems for anticancer agents: an overview and perspectives. Int. J. Nanomedicine 16, 1313–1330 (2021). https://doi.org/10.2147/IJN.S289443
Massoumi, B.; Mossavi, R.; Motamedi, S.; Derakhshankhah, H.; Vandghanooni, S.; Jayman, M.: Fabrication of a dual stimuli-responsive magnetic nanohydrogel for delivery of anticancer drugs. Drug Dev. Ind. Pharm. 47, 1166–111174 (2021). https://doi.org/10.1080/03639045.2021.1988099
Wang, Y.; Chen, L.; Liu, P.: Biocompatible triplex Ag@SiO2@mTiO2 core–shell nanoparticles for simultaneous fluorescence-SERS bimodal imaging and drug delivery. Chem.: Europ. J. 18, 5935–5943 (2012). https://doi.org/10.1002/chem.201103571
El-Fatyany, A.; Wang, H.; Abd El-atty, S.M.: Efficient framework analysis for targeted drug delivery based on internet of bio-nano things. Arab. J. Sci. Eng. 46, 9965–9980 (2021). https://doi.org/10.1007/s13369-021-05651-2
Baig, N.; Kammakakam, I.; Falath, W.: Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2, 1821–1871 (2021). https://doi.org/10.1039/D0MA00807A
Mirza, A.Z.; Siddiqui, F.A.: Nanomedicine and drug delivery: a mini review. Int. Nano Lett. 4, 94–102 (2014). https://doi.org/10.1007/s40089-014-0094-7
Frey, N.A.; Peng, S.; Cheng, K.; Sun, S.: Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 38, 2532–2542 (2009). https://doi.org/10.1039/B815548H
Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S.: Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 16, 1–43 (2015). https://doi.org/10.1088/1468-6996/16/2/023501
Khan, I.; Khalil, A.; Khanday, F.; Shemsi, A.M.; Qurashi, A.; Siddiqui, K.S.: Synthesis, characterization and applications of magnetic iron oxide nanostructures. Arab. J. Sci. Eng. 43, 43–61 (2018). https://doi.org/10.1007/s13369-017-2835-1
Zhu, J.; He, J.; Du, X.; Lu, R.; Huang, L.; Ge, X.: A facile and flexible process of β-cyclodextrin grafted on Fe3O4 magnetic nanoparticles and host-guest inclusion studies. Appl. Surf. Sci. 257, 9056–9062 (2011). https://doi.org/10.1016/J.APSUSC.2011.05.099
Gupta, A.K.; Gupta, M.: Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4002 (2015). https://doi.org/10.1016/j.biomaterials.2004.10.012
Ulbrich, K.; Holá, K.; Šubr, V.; Tuček, J.; Zbořil, R.: Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 116, 5338–5431 (2016). https://doi.org/10.1021/acs.chemrev.5b00589
Yan, H.; Zhang, J.; You, C.; Song, Z.; Yu, B.; Shen, Y.: Surface modification of Fe3O4 nanoparticles and their magnetic properties. Int. J. Miner. Metall. Mater. 16, 226–229 (2009). https://doi.org/10.1016/S1674-4799(09)60038-8
Gupta, A.K.; Gupta, M.: Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4201 (2005). https://doi.org/10.1016/j.biomaterials.2004.10.012
Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H.: Fe3O4@SiO2 Core/shell nanoparticles: the silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 24, 4572–4580 (2012). https://doi.org/10.1021/cm302828d
Chae, H.S.; Kim, D.; Piao, S.H.; Choi, H.J.: Core-shell structured Fe3O4@SiO2 nanoparticles fabricated by sol-gel method and their magnetorheology. Colloid Polym. Sci. 94, 647–655 (2016). https://doi.org/10.1007/s00396-015-3818-y
Hui, C.; Shen, C.; Tian, J.; Bao, J.; Ding, L.; Li, H.; Tian, C.; Shi, Y.; Gao, X.: Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 3, 701–705 (2011). https://doi.org/10.1039/C0NR00497A
Abeer, M.M.; Rewatkar, P.; Qu, Z.; Talekar, M.; Kleitz, F.; Schmid, R.; Lindén, M.; Kumeria, T.; Popat, A.: Silica nanoparticles: a promising platform for enhanced oral delivery of macromolecules. J. Control. Release 326, 544–555 (2020). https://doi.org/10.1016/j.jconrel.2020.07.021
Ding, C.; Guo, Z.; Xiong, J.: Rational design of a multi-responsive drug delivery platform based on SiO2@PPy@poly(acrylic acid-co-acrylamide). React. Funct. Polym. 137, 88–92 (2019). https://doi.org/10.1016/j.reactfunctpolym.2019.02.002
Li, L.; Gu, Z.; Gu, W.; Liu, L.; Xu, Z.P.: Efficient drug delivery using SiO2-layered double hydroxide nanocomposites. J. Colloid Interf. Sci. 470, 47–55 (2016). https://doi.org/10.1016/j.jcis.2016.02.042
Wang, Y.; Zhang, Z.; Abo-zeid, Y.: SiO2-coated layered gadolinium hydroxides for simultaneous drug delivery and magnetic resonance imaging. J. Solid State Chem. 286, 121291–121235 (2020). https://doi.org/10.1016/j.jssc.2020.121291
Zhu, Y.; Tao, C.: DNA-capped Fe3O4/SiO2 magnetic mesoporous silica nanoparticles for potential controlled drug release and hyperthermia. RSC Adv. 5, 22365–22371 (2015). https://doi.org/10.1039/C5RA00701A
Deng, H.; Lei, Z.: Preparation and characterization of hollow Fe3O4/SiO2@PEG–PLA nanoparticles for drug delivery. Compos. Part B: Eng. 54, 194–199 (2013). https://doi.org/10.1016/j.compositesb.2013.05.010
Liu, X.; Tao, Y.; Mao, H.; Kong, Y.; Shen, J.; Deng, L.; Yang, L.: Construction of magnetic-targeted and NIR irradiation-controlled drug delivery platform with Fe3O4@Au@SiO2 nanospheres. Ceram. Int. 43, 5061–5067 (2017). https://doi.org/10.1016/j.ceramint.2017.01.017
Molaei, M.J.; Salimi, E.: Magneto-fluorescent superparamagnetic Fe3O4@SiO2@alginate/carbon quantum dots nanohybrid for drug delivery. Mater. Chem. Phys. 288, 126361–126269 (2022). https://doi.org/10.1049/mnl.2013.0086
Romdoni, Y.; Kadja, T.M.; Kitamoto, Y.; Khalil, M.: Synthesis of multifunctional Fe3O4@SiO2–Ag nanocomposite for antibacterial and anticancer drug delivery. Appl. Surf. Sci. 610, 155610–155618 (2023). https://doi.org/10.1016/j.apsusc.2022.155610
Toomari, Y.; Namazi, H.; Entezami, A.A.: Synthesis of the dendritic type β-cyclodextrin on primary face via click reaction applicable as drug nanocarrier. Carbohyd. Polym. 132, 205–213 (2015). https://doi.org/10.1016/j.carbpol.2015.05.087
Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D.: Improving delivery of hydrophobic drugs from hydrogels through cyclodextrins. J. Biomed. Mater. Res. Part A 74, 454–460 (2005). https://doi.org/10.1002/jbm.a.30374
Xu, J.; Li, X.; Sun, F.: Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release. Acta Biomater. 6, 486–493 (2010). https://doi.org/10.1016/j.actbio.2009.07.021
Machín, R.; Isasi, J.R.; Vélaz, I.: β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohyd. Polym. 87, 2024–2030 (2012). https://doi.org/10.1016/j.carbpol.2011.10.024
Loftsson, T.: Self-assembled cyclodextrin nanoparticles and drug delivery. J. Incl. Phenom. Macrocycl. Chem. 80, 1–7 (2014). https://doi.org/10.1007/s10847-013-0375-1
Chen, Y.Z.: Novel nanoparticles composed of chitosan and β-cyclodextrin derivatives as potential insoluble drug carrier. Chin Chem. Lett. 26, 909–913 (2015). https://doi.org/10.1016/j.cclet.2015.05.044
Liu, C.; Zhang, Z.; Liu, X.: Gelatin-based hydrogels with β-cyclodextrin as a dual functional component for enhanced drug loading and controlled release. RSC Adv. 3, 25041–25049 (2013). https://doi.org/10.1039/C3RA42532K
Ajkidkarn, P.; Ritprajak, P.; Injumpa, W.; Porntaveetus, T.; Insin, N.: Synthesis, characterization, drug release and transdentinal delivery studies of magnetic nanocubes coated with biodegradable poly(2-(dimethyl amino)ethyl methacrylate). J. Magnet. Magnet. Mater. 427, 235–240 (2017). https://doi.org/10.1016/j.jmmm.2016.11.020
Pourjavadi, A.; Tehrani, Z.M.: Poly(N-isopropylacrylamide)-coated β-cyclodextrin-capped magnetic mesoporous silica nanoparticles exhibiting thermal and pH dual response for triggered anticancer drug delivery. Int. J. Polym. Mater. Polym. Biomater. 66, 336–348 (2017). https://doi.org/10.1080/00914037.2016.1217531
Tang, W.; Zhao, J.; Sha, B.; Liu, H.: Adsorption and drug release based on β-cyclodextrin-grafted hydroxyapatite composite. J. Appl. Polym. Sci. 127, 2803–2808 (2013). https://doi.org/10.1002/app.37607
Mbituyimana, B.; Ma, G.; Shi, Z.; Yang, G.: Polymer-based microneedle composites for enhanced non-transdermal drug delivery. Appl. Mater. Today 29, 101659–101667 (2022). https://doi.org/10.1016/j.apmt.2022.101659
Amoyav, B.; Goldstein, Y.; Steinberg, E.: 3D Printed microfluidic devices for drug release assays. Pharmaceutics 13, 13–27 (2021). https://doi.org/10.3390/pharmaceutics13010013
Yadav, H.; Agrawal, R.; Panday, A.; Patel, J.; Maiti, S.: Polysaccharide-silicate composite hydrogels: review on synthesis and drug delivery credentials. J. Drug Deliv. Sci. Technol. 74, 103573–103579 (2022). https://doi.org/10.1016/j.jddst.2022.103573
Wang, F.; Huang, K.; Xu, Z.; Shi, F.; Chen, C.: Self-healable nanocellulose composite hydrogels combining multiple dynamic bonds for drug delivery. Int. J. Biolog. Macromol. 203, 143–152 (2022). https://doi.org/10.1016/j.ijbiomac.2022.01.127
Zhang, X.; An, D.; Zhang, R.; Huang, Y.; Liu, Z.: Preparation of carbon nanotubes and polyhedral oligomeric-reinforced molecularly imprinted polymer composites for drug delivery of gallic acid. Int. J. Pharmac. 615, 121476–121483 (2022). https://doi.org/10.1016/j.ijpharm.2022.121476
Ojagh, S.M.A.; Vahabzadeh, F.; Kari, A.: Synthesis and characterization of bacterial cellulose-based composites for drug delivery. Carbohyd. Polym. 273, 118587–118593 (2021). https://doi.org/10.1016/j.carbpol.2021.118587
Agrahari, V.: Novel drug delivery systems, devices, and fabrication methods. Drug Deliv. Transl. Res. 8, 303–306 (2018). https://doi.org/10.1007/s13346-017-0459-3
Zhaoz, Y.; Qiu, Z.; Huang, J.: Preparation and analysis of magnetic nanoparticles used as targeted-drug carriers. Chin. J. Chem. Eng. 16, 451–455 (2008). https://doi.org/10.1016/S1004-9541(08)60104-4
Nakabayashi, K.; Mori, H.: Recent progress in controlled radical polymerization of N-vinyl monomers. Europ. Polym. J. 49, 2808–2838 (2013). https://doi.org/10.1016/j.eurpolymj.2013.07.006
Pooresmaeil, M.; Namazi, H.: β-Cyclodextrin grafted magnetic graphene oxide applicable as cancer drug delivery agent: synthesis and characterization. Mater. Chem. Phys. 218, 62–69 (2018). https://doi.org/10.1016/j.matchemphys.2018.07.022
Mohamed, M.H.; Wilson, L.D.; Headley, J.V.: Design and characterization of novel β-cyclodextrin based copolymer materials. Carbohyd. Res. 346, 219–229 (2011). https://doi.org/10.1016/j.carres.2010.11.022
BabuL, K.; Reddy, Y.V.: Synthesis and characterization of magnetically core-shell structured CoFe2O4/SiO2 nanoparticles; their enhanced antibacterial and electrocatalytic properties. Colloids Surf. A: Physicochem. Eng. Asp. 598, 124806–124816 (2020). https://doi.org/10.1016/j.colsurfa.2020.124806
Poor Heravi, M.R.; Aghamohammadi, P.; Vessally, E.: Green synthesis and antibacterial, antifungal activities of 4H-pyran, tetrahydro-4H-chromenes and spiro2-oxindole derivatives by highly efficient Fe3O4@SiO2@NH2@Pd(OCOCH3)2 nanocatalyst. J. Mol. Struct. 1249, 131534–131539 (2022). https://doi.org/10.1016/j.molstruc.2021.131534
Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E.: Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 96, 968–975 (2016). https://doi.org/10.1016/j.foodchem.2015.10.023
Zhou, L.; Gao, C.; Hu, X.; Xu, W.: Fe3O4/SiO2-Pt/Au/Pd magnetic nanocatalysts with multifunctional hyperbranched polyglycerol amplifiers. Langmuir 26, 11217–11225 (2010). https://doi.org/10.1021/la100556p
Taherian, A.; Esfandiari, N.; Rouhani, S.: Breast cancer drug delivery by novel drug-loaded chitosan-coated magnetic nanoparticles. Cancer Nanotechnol. 12, 15–34 (2021). https://doi.org/10.1186/s12645-021-00086-8
Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U.: Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 62, 243–250 (2014). https://doi.org/10.1016/j.ejps.2014.05.021
Shirke, Y.M.; Abou-Elanwar, A.M.; Kwon, S.J.: Development of nanocomposite membranes based on sulfated β-cyclodextrin/glutaraldehyde with magnetically recoverable magnetite-carbon dot hybrid nanoparticles for water vapor dehumidification. J. Environ. Chem. Eng. 10, 107042–107049 (2022). https://doi.org/10.1016/j.jece.2021.107042
Wang, H.; Zhang, C.; Zhang, X.: Construction of Fe3O4@β-CD/g-C3N4 nanocomposite catalyst for degradation of PCBs in wastewater through photodegradation and heterogeneous Fenton oxidation. Chem. Eng. J. 429, 132445–1322457 (2022). https://doi.org/10.1016/j.cej.2021.132445
Bosu, S.; Rajamohan, N.; Rajasimman, M.: Enhanced remediation of lead (II) and cadmium (II) ions from aqueous media using porous magnetic nanocomposites-a comprehensive review on applications and mechanism. Environ. Res. 213, 113720–113729 (2022). https://doi.org/10.1016/j.envres.2022.113720
Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Yin, C.: Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles. RSC Adv. 8, 24633–24640 (2018). https://doi.org/10.1039/C8RA03914C
Li, Z.; Liu, D.; Cai, Y.; Wang, Y.; Teng, J.: Adsorption pore structure and its fractal characteristics of coals by N2 adsorption/desorption and FESEM image analyses. Fuel 257, 116031–116043 (2019). https://doi.org/10.1016/j.fuel.2019.116031
Rasouli, S.; Davaran, S.; Rasouli, F.: Synthesis, characterization and pH-controllable methotrexate release from biocompatible polymer/silica nanocomposite for anticancer drug delivery. Drug Deliv. 21, 155–163 (2013). https://doi.org/10.3109/10717544.2013.838714
Fu, Y.; Kao, W.J.: Drug release kinetics and transport mechanisms of nondegradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 7, 429–444 (2010). https://doi.org/10.1517/17425241003602259
Modi, S.; Anderso, B.D.: Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol. Pharmac. 10, 3076–3089 (2013). https://doi.org/10.1021/mp400154a
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseinzadeh, H., Jahanbakhsh, Z., Masoumi, B. et al. Preparation of Amino-Functionalized β-Cyclodextrin/Fe3O4@SiO2 Magnetic Nanocarrier for Controlled Release of Doxorubicin, an Anticancer Drug. Arab J Sci Eng 49, 459–473 (2024). https://doi.org/10.1007/s13369-023-08202-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08202-z