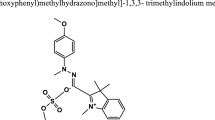
Abstract
In this study, the treatment of aqueous media containing Astrazon Pink FG (AFG) dye, widely used in the textile industry but with limited studies, was investigated using the Fenton process. The system was numerically optimized as Fe2+: 50 mg/L, H2O2: 50 mg/L, pH 3.75, reaction time: 42.54 min, and initial dye concentration: 100 mg/L based on the principle of low-cost high removal efficiency. The quadratic model with central composite design was reliable, valid, and significant (p < 0.0001) for both system responses Theoretical removal efficiencies under these conditions were determined as 80.5% and 94.11% for chemical oxygen demand (COD) and AFG removal, respectively, and were confirmed experimentally as 81.01% and 94.33% under the same conditions. The performance of the Fenton process under optimized conditions was calculated as 51%, 65%, and 73% for COD, AFG and Methyl Orange removal. Reactive Yellow 86, Acid Orange 7, and Reactive Green 19 were removed as 62.72%, 51.73%, and 39.39%, respectively, from real textile wastewater. The generated sludges (v/v) under optimized conditions for AFG dye solution, binary dye solution and real textile wastewater were 6%, 5% and 7%, respectively. AFG removal best fitted the BMG model (R2 > 0.998). According to the experimental cost estimation based on chemical consumption under optimized conditions, 1 m3 of AFG solution can be treated at $0.26. It was concluded that the Fenton process could be used as a pretreatment for industrial wastewater containing dye.




Similar content being viewed by others
References
Easton, J. R., Waters, B. D., Churchley, J. H., Harrison, J.: Colour in dyehouse effluent. Society of Dyers and Colourists (1995)
Elkady, M.F.; Ibrahim, A.M.; El-Latif, M.M.A.: Assessment of the adsorption kinetics, equilibrium and thermodynamic for the potential removal of reactive red dye using eggshell biocomposite beads. Desalination (2011). https://doi.org/10.1016/j.desal.2011.05.063
Ozturk, D.; Sahan, T.; Bayram, T.; Erkus, A.: Application of response surface methodology (RSM) to optimize the adsorption conditions of cationic basic yellow 2 onto pumice samples as a new adsorbent. Fresenius Environ. Bull. 26, 3285–3292 (2017)
Khan, I.; Luo, M.; Guo, L.; Khan, S.; Shah, S.A.; Khan, I.; Khan, A.; Wang, C.; Ai, B.; Zaman, S.: Synthesis of phosphate-bridged g-C3N4/LaFeO3 nanosheets Z-scheme nanocomposites as efficient visible photocatalysts for CO2 reduction and malachite green degradation. Appl. Catal. A Gen. 629, 118418 (2022). https://doi.org/10.1016/j.apcata.2021.118418
Yönten, V.; Sanyürek, N.K.; Kivanç, M.R.: A thermodynamic and kinetic approach to adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Vitis vinifera L. Surf. Interfaces. 20, 100529 (2020). https://doi.org/10.1016/j.surfin.2020.100529
Castro, F.D.; Bassin, J.P.; Alves, T.L.M.; Sant’Anna, G.L.; Dezotti, M.: Reactive Orange 16 dye degradation in anaerobic and aerobic MBBR coupled with ozonation: addressing pathways and performance. Int. J. Environ. Sci. Technol. 18, 1991–2010 (2021). https://doi.org/10.1007/s13762-020-02983-8
Krishnan, J.; Arvind Kishore, A.; Suresh, A.; Madhumeetha, B.; Gnana Prakash, D.: Effect of pH, inoculum dose and initial dye concentration on the removal of azo dye mixture under aerobic conditions. Int. Biodeterior. Biodegrad. 119, 16–27 (2017). https://doi.org/10.1016/j.ibiod.2016.11.024
Daneshvar, N.; Oladegaragoze, A.; Djafarzadeh, N.: Decolorization of basic dye solutions by electrocoagulation: an investigation of the effect of operational parameters. J. Hazard. Mater. (2006). https://doi.org/10.1016/j.jhazmat.2005.08.033
Nery, V. Del; Nardi, I. De; Resources, M.D.-; conservation, undefined; 2007, undefined: Long-term operating performance of a poultry slaughterhouse wastewater treatment plant. Elsevier.
Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B.: Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 42, 102122 (2021). https://doi.org/10.1016/j.jwpe.2021.102122
Hussain, S.M.; Hussain, T.; Faryad, M.; Ali, Q.; Ali, S.; Rizwan, M.; Hussain, A.I.; Ray, M.B.; Chatha, S.A.S.: Emerging aspects of photo-catalysts (TiO2 & ZnO) doped zeolites and advanced oxidation processes for degradation of azo dyes: a review Curr. Anal. Chem. 17(82), 97 (2020). https://doi.org/10.2174/1573411016999200711143225
Ozturk, D.; Yilmaz, A.E.: Investigation of electrochemical degradation of Basic Red 13 dye in aqueous solutions based on COD removal: numerical optimization approach. Int. J. Environ. Sci. Technol. 17, 3099–3110 (2020). https://doi.org/10.1007/s13762-020-02692-2
Quanfang, L.; Jie, Y.; Cailing, Y.; Minrui, L.: Degradation mechanism of Astrazon Pink FG solution by glow discharge electrolysis. CIESC J. 69, 2664–2671 (2018). https://doi.org/10.11949/j.issn.0438-1157.20171310
Sen Gupta, S.K.: Contact glow discharge electrolysis: its origin, plasma diagnostics and non-faradaic chemical effects. Plasma Sources Sci. Technol. 24, 063001 (2015). https://doi.org/10.1088/0963-0252/24/6/063001
Hasija, V.; Nguyen, V.H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A.A.P.; Singh, P.; Lichtfouse, E.; Wang, C.; Huong, P.T.: Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: a review. J. Hazard. Mater. 413, 125324 (2021). https://doi.org/10.1016/j.jhazmat.2021.125324
Bhat, A.P.; Gogate, P.R.: Degradation of nitrogen-containing hazardous compounds using advanced oxidation processes: A review on aliphatic and aromatic amines, dyes, and pesticides. J. Hazard. Mater. 403, 123657 (2021). https://doi.org/10.1016/j.jhazmat.2020.123657
De León, M.A.; Sergio, M.; Bussi, J.: Iron-pillared clays as catalysts for dye removal by the heterogeneous photo-Fenton technique. React. Kinet. Mech. Catal. 110, 101–117 (2013). https://doi.org/10.1007/s11144-013-0593-y
Ozturk, D.: Fe3O4/Mn3O4/ZnO-rGO hybrid quaternary nano-catalyst for effective treatment of tannery wastewater with the heterogeneous electro-Fenton process: process optimization. Sci. Total Environ. 828, 154473 (2022). https://doi.org/10.1016/j.scitotenv.2022.154473
Sudhaik, A.; Parwaz Khan, A.A.; Raizada, P.; Nguyen, V.-H.; Van Le, Q.; Asiri, A.M.; Singh, P.: Strategies based review on near-infrared light-driven bismuth nanocomposites for environmental pollutants degradation. Chemosphere. 291, 132781 (2021). https://doi.org/10.1016/j.chemosphere.2021.132781
Manea, Y.K.; Khan, A.M.; Wani, A.A.; Saleh, M.A.S.; Qashqoosh, M.T.A.; Shahadat, M.; Rezakazemi, M.: In-grown flower like Al-Li/Th-LDH@CNT nanocomposite for enhanced photocatalytic degradation of MG dye and selective adsorption of Cr (VI). J. Environ. Chem. Eng. 10, 106848 (2022). https://doi.org/10.1016/j.jece.2021.106848
Bhaumik, M.; Maity, A.; Brink, H.G.: Metallic nickel nanoparticles supported polyaniline nanotubes as heterogeneous Fenton-like catalyst for the degradation of brilliant green dye in aqueous solution. J. Colloid Interface Sci. 611, 408–420 (2022). https://doi.org/10.1016/j.jcis.2021.11.181
Mahmud, N.; Benamor, A.; Nasser, M.S.; Ba-Abbad, M.M.; El-Naas, M.H.; Mohammad, A.W.: Effective heterogeneous fenton-like degradation of malachite green dye using the core-shell Fe3O4 @SiO2 nano-catalyst. ChemistrySelect 6, 865–875 (2021). https://doi.org/10.1002/slct.202003937
Modarresi-Motlagh, S.; Bahadori, F.; Ghadiri, M.; Afghan, A.: Enhancing Fenton-like oxidation of crystal violet over Fe/ZSM-5 in a plug flow reactor. React. Kinet. Mech. Catal. 133, 1061–1073 (2021). https://doi.org/10.1007/s11144-021-02001-z
Tabaï, A.; Bechiri, O.; Abbessi, M.: Degradation of organic dye using a new homogeneous Fenton-like system based on hydrogen peroxide and a recyclable Dawson-type heteropolyanion. Int. J. Ind. Chem. 8, 83–89 (2017). https://doi.org/10.1007/s40090-016-0104-x
Modak, J.B.; Bhowal, A.; Datta, S.; Karmakar, S.: Continuous decolorization of dye solution by homogeneous Fenton process in a rotating packed bed reactor. Int. J. Environ. Sci. Technol. 17, 1691–1702 (2020). https://doi.org/10.1007/s13762-019-02548-4
Lalwani, J.; Thatikonda, S.; Challapalli, S.: Varying efficacies of Fenton’s oxidation treatment on pharmaceutical industry effluents of contrasting viscosity profiles. CLEAN Soil, Air, Water 49(3), 2000335 (2021). https://doi.org/10.1002/clen.202000335
Şahan, T.; Öztürk, D.: Investigation of Pb(II) adsorption onto pumice samples: application of optimization method based on fractional factorial design and response surface methodology. Clean Technol. Environ. Policy. 16, 819–831 (2014). https://doi.org/10.1007/s10098-013-0673-8
Mandal, T.; Maity, S.; Dasgupta, D.; Datta, S.: Advanced oxidation process and biotreatment: their roles in combined industrial wastewater treatment. Desalination 250, 87–94 (2010). https://doi.org/10.1016/j.desal.2009.04.012
Eaton, A.: Measuring UV-absorbing organics: a standard method. J. Am. Water Works Assoc. (1995). https://doi.org/10.1002/j.1551-8833.1995.tb06320.x
Ekmekyapar Torun, F.; Cengiz, İ.; Kul, S.: Investigation of olive mill wastewater treatment with advanced oxidation processes. J. Inst. Sci. Technol. 1597–1606 (2020). https://doi.org/10.21597/jist.687345
Talinli, I.; Anderson, G.K.: Interference of hydrogen peroxide on the standard cod test. Water Res. 26, 107–110 (1992). https://doi.org/10.1016/0043-1354(92)90118-N
Benatti, C.T.; Tavares, C.R.G.; Guedes, T.A.: Optimization of Fenton’s oxidation of chemical laboratory wastewaters using the response surface methodology. J. Environ. Manage. 80, 66–74 (2006). https://doi.org/10.1016/j.jenvman.2005.08.014
Hatami, M.; Domairry, G.; Mirzababaei, S.N.: Experimental investigation of preparing and using the H 2 O based nanofluids in the heating process of HVAC system model. Int. J. Hydrogen Energy. 42, 7820–7825 (2017). https://doi.org/10.1016/j.ijhydene.2016.12.104
Ighalo, J.O.; Adeniyi, A.G.; Eletta, O.A.A.; Ojetimi, N.I.; Ajala, O.J.: Evaluation of Luffa cylindrica fibres in a biomass packed bed for the treatment of fish pond effluent before environmental release. Sustain. Water Resour. Manag. 6, 120 (2020). https://doi.org/10.1007/s40899-020-00485-6
Aniyikaiye, T.; Oluseyi, T.; Odiyo, J.; Edokpayi, J.: Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health. 16, 1235 (2019). https://doi.org/10.3390/ijerph16071235
APHA Awwa WEF: Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC (2012)
Punzi, M.; Mattiasson, B.; Jonstrup, M.: Treatment of synthetic textile wastewater by homogeneous and heterogeneous photo-Fenton oxidation. J. Photochem. Photobiol. A Chem. 248, 30–35 (2012). https://doi.org/10.1016/j.jphotochem.2012.07.017
Dada, M.; Popoola, P.; Mathe, N.; Pityana, S.; Adeosun, S.: Parametric optimization of laser deposited high entropy alloys using response surface methodology (RSM). Int. J. Adv. Manuf. Technol. 109, 2719–2732 (2020). https://doi.org/10.1007/s00170-020-05781-1
Beldjoudi, S.; Kouachi, K.; Bourouina-Bacha, S.; Bouchene, H.; Deflaoui, O.; Lafaye, G.: Experimental and theoretical investigation of a homogeneous Fenton process for the degradation of an azo dye in batch reactor. React. Kinet. Mech. Catal. 133, 139–155 (2021). https://doi.org/10.1007/s11144-021-01979-w
Öztürk, D.; Şahan, T.: Design and optimization of Cu(II) adsorption conditions from aqueous solutions by low-cost adsorbent pumice with response surface methodology. Polish J. Environ. Stud. 24, 1749–1756 (2015). https://doi.org/10.15244/pjoes/40270
Ozturk, D.; Dagdas, E.; Fil, B.; Bashir, M.J.K.: Central composite modeling for electrochemical degradation of paint manufacturing plant wastewater: one-step/two-response optimization. Environ. Technol. Innov. 21, 101264 (2020). https://doi.org/10.1016/j.eti.2020.101264
Kıvanç, M.R.; Yönten, V.: A statistical optimization of methylene blue removal from aqueous solutions by Agaricus Campestris using multi-step experimental design with response surface methodology: Isotherm, kinetic and thermodynamic studies. Surf. Interfaces. 18, 100414 (2020). https://doi.org/10.1016/j.surfin.2019.100414
Karimi, B.; Rokhzadi, A.; Rahimi, A.R.: RSM modeling of nitrogen use efficiency, biomass and essential oil of Salvia officinalis L. as affected by fertilization and plant density. J. Plant Nutr. 44, 1067–1084 (2021). https://doi.org/10.1080/01904167.2021.1871756
Islam, A.; Chauhan, A.; Javed, H.; Rais, S.; Ahmad, I.: Magnetic carbon nanotubes-silica binary composite for effective Pb (II) sequestration from industrial effluents: multivariate process optimization. CLEAN Soil, Air, Water 49(8), 2000401 (2021). https://doi.org/10.1002/clen.202000401
Sridhar, R.; Sivakumar, V.; Thirugnanasambandham, K.: Response surface modeling and optimization of upflow anaerobic sludge blanket reactor process parameters for the treatment of bagasse based pulp and paper industry wastewater. Desalin. Water Treat. 1–12 (2015). https://doi.org/10.1080/19443994.2014.999712
Radhwan, H.; Shayfull, Z.; Farizuan, M.R.; Effendi, M.S.M.; Irfan, A.R.: Optimization parameter effects on the quality surface finish of the three-dimensional printing (3D-printing) fused deposition modeling (FDM) using RSM. Presented at the (2019)
Bhattacharya, S.S.; Banerjee, R.: Laccase mediated biodegradation of 2,4-dichlorophenol using response surface methodology. Chemosphere (2008). https://doi.org/10.1016/j.chemosphere.2008.05.005
Barahimi, V.; Mehrabani-Zeinabad, A.; Rahmati, M.; Ghafaripoor, M.: Synthesis, characterization, and evaluations of Cu-doped TiO2/Bi2O3 nanocomposite for Direct red 16 azo dye decolorization under visible light irradiation. Desalin. WATER Treat. 202, 450–461 (2020). https://doi.org/10.5004/dwt.2020.26165
Manmai, N.; Unpaprom, Y.; Ramaraj, R.: Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Convers. Biorefinery. 11, 1759–1773 (2021). https://doi.org/10.1007/s13399-020-00602-7
Prasath, K.M.; Pradheep, T.; Suresh, S.: Application of Taguchi and response surface methodology (RSM) in steel turning process to improve surface roughness and material removal rate. Mater. Today Proc. 5, 24622–24631 (2018). https://doi.org/10.1016/j.matpr.2018.10.260
Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S.: Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 25, 317–321 (2016). https://doi.org/10.1016/j.ejpe.2015.07.017
Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y.: Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 58, 1409–1414 (2005). https://doi.org/10.1016/j.chemosphere.2004.09.091
Sun, S.-P.; Li, C.-J.; Sun, J.-H.; Shi, S.-H.; Fan, M.-H.; Zhou, Q.: Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: Effect of system parameters and kinetic study. J. Hazard. Mater. 161, 1052–1057 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.080
Kwon, B.G.; Lee, D.S.; Kang, N.; Yoon, J.: Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Res. 33, 2110–2118 (1999). https://doi.org/10.1016/S0043-1354(98)00428-X
Toprak, D.; Sener, S. (2021) Sentetik tekstil atıksuyunun fenton ve ultrases-fenton oksidasyon yöntemleri ile renk ve Koi gideriminin araştırılması. Ömer Halisdemir Üniversitesi Mühendislik Bilim. Derg https://doi.org/10.28948/ngumuh.749438
Bayhan, Y.K.; Değermenci, G.D.: Kozmetik atık sularından fenton prosesiyle organik madde gideriminin ve kinetiğinin incelenmesi. Gazi Üniversitesi Mühendislik-Mimarlık Fakültesi Derg https://doi.org/10.17341/gazimmfd.300609
Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L.: Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—a review. Chemosphere 174, 665–688 (2017). https://doi.org/10.1016/j.chemosphere.2017.02.019
Güneş, E.; Cihan, M.T.: COD and color removal from wastewaters: optimization of Fenton process. Pamukkale Univ. J. Eng. Sci. 21, 239–247 (2015). https://doi.org/10.5505/pajes.2014.37928
Alalm, M.G.; Tawfik, A.; Ookawara, S.: Degradation of four pharmaceuticals by solar photo-Fenton process: kinetics and costs estimation. J. Environ. Chem. Eng. 3, 46–51 (2015). https://doi.org/10.1016/j.jece.2014.12.009
Zhang, H.; Fu, H.; Zhang, D.: Degradation of C.I. Acid Orange 7 by ultrasound enhanced heterogeneous Fenton-like process. J. Hazard. Mater. 172, 654–660 (2009). https://doi.org/10.1016/j.jhazmat.2009.07.047
Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Shirakashi, T.: Mineralization of bisphenol A (BPA) by anodic oxidation with boron-doped diamond (BDD) electrode. J. Hazard. Mater. 154, 213–220 (2008). https://doi.org/10.1016/j.jhazmat.2007.10.011
Nguyen, L.H.; Van, H.T.; Ngo, Q.N.; Thai, V.N.; Hoang, V.H.; Hai, N.T.T.: Improving Fenton-like oxidation of Rhodamin B using a new catalyst based on magnetic/iron-containing waste slag composite. Environ. Technol. Innov. 23, 101582 (2021). https://doi.org/10.1016/j.eti.2021.101582
Rodrigues, C.S.D.; Madeira, L.M.; Boaventura, R.A.R.: Optimization of the azo dye Procion Red H-EXL degradation by Fenton’s reagent using experimental design. J. Hazard. Mater. 164, 987–994 (2009). https://doi.org/10.1016/J.JHAZMAT.2008.08.109
Shi, X.; Tian, A.; You, J.; Yang, H.; Wang, Y.; Xue, X.: Degradation of organic dyes by a new heterogeneous Fenton reagent - Fe2GeS4 nanoparticle. J. Hazard. Mater. 353, 182–189 (2018). https://doi.org/10.1016/j.jhazmat.2018.04.018
Öztürk, D.: Degradation of Reactive Orange 16 dye with heterogeneous Fenton Process using magnetic nano-sized clay as catalyst: a central composite optimization study. Hacettepe J. Biol. Chem. 50, 113–129 (2021). https://doi.org/10.15671/hjbc.937728
Giwa, A.-R.A.; Bello, I.A.; Olabintan, A.B.; Bello, O.S.; Saleh, T.A.: Kinetic and thermodynamic studies of fenton oxidative decolorization of methylene blue. Heliyon. 6, e04454 (2020). https://doi.org/10.1016/j.heliyon.2020.e04454
Suhan, M.B.K.; Mahtab, S.M.T.; Aziz, W.; Akter, S.; Islam, M.S.: Sudan black B dye degradation in aqueous solution by Fenton oxidation process: kinetics and cost analysis. Case Stud. Chem. Environ. Eng. 4, 100126 (2021). https://doi.org/10.1016/J.CSCEE.2021.100126
Alıasgharlou, N.; Bahram, M.; Zolfagharı, P.; Mohsenı, N.: Modeling and optimization of simultaneous degradation of rhodamine B and acid red 14 binary solution by homogeneous Fenton reaction: a chemometrics approach. Turkish J. Chem. 44, 987–1001 (2020). https://doi.org/10.3906/kim-2002-59
Behnajady, M.A.; Modirshahla, N.; Ghanbary, F.: A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater. 148, 98–102 (2007). https://doi.org/10.1016/j.jhazmat.2007.02.003
Hashemian, S.: Fenton-like oxidation of malachite green solutions: kinetic and thermodynamic study. J. Chem. 2013, 1–7 (2013). https://doi.org/10.1155/2013/809318
Sidney Santana, C.; Nicodemos Ramos, M.D.; Vieira Velloso, C.C.; Aguiar, A.: Kinetic Evaluation of dye decolorization by fenton processes in the presence of 3-hydroxyanthranilic acid. Int. J. Environ. Res. Public Health. 16, 1602 (2019). https://doi.org/10.3390/ijerph16091602
Lima, J.P.P.; Tabelini, C.H.B.; Ramos, M.D.N.; Aguiar, A.: Kinetic evaluation of bismarck brown y azo dye oxidation by Fenton processes in the presence of aromatic mediators. Water, Air, Soil Pollut. 232, 321 (2021). https://doi.org/10.1007/s11270-021-05258-1
Jafari, S.; Dehghani, M.; Nasirizadeh, N.; Akrami, H.R.: Voltammetric determination of Basic Red 13 during its sonoelectrocatalytic degradation. Microchim. Acta. 184, 4459–4468 (2017). https://doi.org/10.1007/s00604-017-2482-y
Beldjoudi, S.; Kouachi, K.; Bourouina-Bacha, S.; Lafaye, G.; Soualah, A.: Kinetic study of methyl orange decolorization by the Fenton process based on fractional factorial design. React. Kinet. Mech. Catal. 130, 1123–1140 (2020). https://doi.org/10.1007/s11144-020-01803-x
da Santana, R.M.; Napoleão, D.C.; Duarte, M.M.M.B.: Treatment of textile matrices using Fenton processes influence of operational parameters on degradation kinetics, ecotoxicity evaluation and application in real wastewater. J. Environ. Sci. Heal. Part A. 56, 1165–1178 (2021). https://doi.org/10.1080/10934529.2021.1965816
Babuponnusami, A.; Muthukumar, K.: Degradation of phenol in aqueous solution by Fenton, Sono-fenton and Sono-photo-fenton methods. CLEAN Soil, Air, Water. 39, 142–147 (2011). https://doi.org/10.1002/clen.201000072
Li, H.; Li, Y.; Xiang, L.; Huang, Q.; Qiu, J.; Zhang, H.; Sivaiah, M.V.; Baron, F.; Barrault, J.; Petit, S.; Valange, S.: Heterogeneous photo-Fenton decolorization of Orange II over Al-pillared Fe-smectite: response surface approach, degradation pathway, and toxicity evaluation. J. Hazard. Mater. 287, 32–41 (2015). https://doi.org/10.1016/j.jhazmat.2015.01.023
Zhang, Q.; Wang, C.; Lei, Y.: Fenton’s oxidation kinetics, pathway, and toxicity evaluation of diethyl phthalate in aqueous solution. J. Adv. Oxid. Technol. (2016). https://doi.org/10.1515/jaots-2016-0117
Ertugay, N.; Acar, F.N.: Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: Kinetic study. Arab. J. Chem. 10, S1158–S1163 (2017). https://doi.org/10.1016/j.arabjc.2013.02.009
Khan, N.-U.-H.; Bhatti, H.N.; Iqbal, M.; Nazir, A.: Decolorization of Basic Turquise Blue X-GB and Basic Blue X-GRRL by the Fenton’s Process and its Kinetics. Zeitschrift für Phys. Chemie. 233, 361–373 (2019). https://doi.org/10.1515/zpch-2018-1194
Tong, S.; Shen, J.; Jiang, X.; Li, J.; Sun, X.; Xu, Z.; Chen, D.: Recycle of Fenton sludge through one-step synthesis of aminated magnetic hydrochar for Pb2+ removal from wastewater. J. Hazard. Mater. 406, 124581 (2021). https://doi.org/10.1016/j.jhazmat.2020.124581
Gao, L.; Cao, Y.; Wang, L.; Li, S.: A review on sustainable reuse applications of Fenton sludge during wastewater treatment. Front. Environ. Sci. Eng. 16, 77 (2022). https://doi.org/10.1007/s11783-021-1511-6
Zhang, H.; Xue, G.; Chen, H.; Li, X.: Hydrothermal synthesizing sludge-based magnetite catalyst from ferric sludge and biosolids: Formation mechanism and catalytic performance. Sci. Total Environ. 697, 133986 (2019). https://doi.org/10.1016/J.SCITOTENV.2019.133986
Wang, G.; Tang, K.; Jiang, Y.; Andersen, H.R.; Zhang, Y.: Regeneration of Fe(II) from Fenton-derived ferric sludge using a novel biocathode. Bioresour. Technol. 318, 124195 (2020). https://doi.org/10.1016/J.BIORTECH.2020.124195
Zhang, H.; Liu, J.; Ou, C.; Shen, J.; Yu, H.; Jiao, Z.; Han, W.; Sun, X.; Li, J.; Wang, L.: Reuse of Fenton sludge as an iron source for NiFe2O4 synthesis and its application in the Fenton-based process. J. Environ. Sci. 53, 1–8 (2017). https://doi.org/10.1016/J.JES.2016.05.010
Bayram, T.; Bucak, S.; Ozturk, D.: BR13 dye removal using sodium dodecyl sulfate modified montmorillonite: equilibrium, thermodynamic, kinetic and reusability studies. Chem. Eng. Process. Process Intensif. 158, 108186 (2020). https://doi.org/10.1016/j.cep.2020.108186
Katsumata, H.; Koike, S.; Kaneco, S.; Suzuki, T.; Ohta, K.: Degradation of Reactive Yellow 86 with photo-Fenton process driven by solar light. J. Environ. Sci. 22, 1455–1461 (2010). https://doi.org/10.1016/S1001-0742(09)60275-8
Liu, H.; Li, G.; Qu, J.; Liu, H.: Degradation of azo dye Acid Orange 7 in water by Fe0/granular activated carbon system in the presence of ultrasound. J. Hazard. Mater. 144, 180–186 (2007). https://doi.org/10.1016/J.JHAZMAT.2006.10.009
Hsueh, C.C.; Chen, B.Y.; Yen, C.Y.: Understanding effects of chemical structure on azo dye decolorization characteristics by Aeromonas hydrophila. J. Hazard. Mater. 167, 995–1001 (2009). https://doi.org/10.1016/J.JHAZMAT.2009.01.077
Singh, S.K.; Tang, W.Z.; Tachiev, G.: Fenton treatment of landfill leachate under different COD loading factors. Waste Manag. 33, 2116–2122 (2013). https://doi.org/10.1016/j.wasman.2013.06.019
Asghar, A.; Abdul Raman, A.A.; Daud, W.M.A.W.: Sequential optimization for minimizing material cost and treatment time of fenton oxidation for textile wastewater treatment. Chem. Eng. Commun. 204, 873–883 (2017). https://doi.org/10.1080/00986445.2017.1320283
Pliego, G.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J.: Case study of the application of Fenton process to highly polluted wastewater from power plant. J. Hazard. Mater. 252–253, 180–185 (2013). https://doi.org/10.1016/j.jhazmat.2013.02.042
Acknowledgements
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Contributions
DO contributed to conceptualization, investigation, experimentation, methodology, software using, data analysis, supervision, validation, visualization, writing–original draft, writing–review and editing, and AO contributed to investigation, experimentation, methodology, data analysis, validation, writing–original draft, writing–review and editing.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ozguven, A., Ozturk, D. A Numerical Optimization Approach for Removal of Astrazon Pink FG from Aqueous Media by Fenton Oxidation. Arab J Sci Eng 48, 8431–8452 (2023). https://doi.org/10.1007/s13369-022-06996-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-06996-y