Abstract
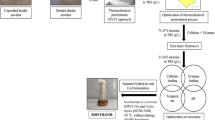
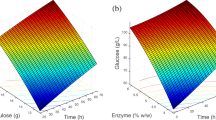
The present paper discusses response surface methodology (RSM) as an efficient tactic for predictive model and optimization of the whole experimental methods of reducing sugar and energy. In this work, the application of RSM presented for optimizing reducing sugar and energy as compared with production between chemical and biological pretreatments. All experiments applied statistical designs in order to develop a statistic multivariate analysis model that provides to consider the effect of different parameters on a process and describe the optimum values of these variables to optimize the response. Dred sunflower stalks were pretreated by sodium hydroxide (NaOH) and Trichoderma reesei as a function of two variables: concentration of NaOH (%) and T. reesei (%) and time for pretreatment (Day) to receive reducing sugar and energy. The chemical pretreatment model was characterized by 13 runs, varying the variables at two factors, NaOH (1, 1.5, 2%) and Day (1, 2, 3). The biological pretreatment model was characterized by 13 runs, varying the variables at two factors, T. reesei (1, 1.5, 2%) and Day (1, 2, 3), by central composite design experimental design. In the chemical pretreatment, experiments performed at 2% (w/v) of NaOH for 3 days were used. The chemical pretreatment model at 2% NaOH for a 3-day release reduced sugar by 5.812 g/L and energy by 92.992 kJ/L; on the other hand, biological pretreatment model at 2% T. reesei for a 3-day release reduced sugar by 3.891 g/L and energy by 62.256 kJ/L, reducing sugar starter for fermentation by 49.0670 ± 6.4660 g/L and fermentation efficiency by 71.60% at 48 h fermented time.










Similar content being viewed by others
References
Ramaraj R, Kawaree R, Unpaprom Y (2015) A newly isolated green alga, Pediastrum duplex Meyen, from Thailand with efficient hydrogen production. IJSGE 4(1–1):7–12
Bchir FS, Falleh AE, Ghabbarou E, Hamdi E (2016) 3rd generation bioethanol production from microalgae isolated from slaughterhouse wastewater. Waste Biomass Valori 7(5):1041–1046
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second-generation biofuels: a comprehensive review. Renew Sust Energ Rev 14(2):578–597
Wannapokin A, Ramaraj R, Whangchai K, Unpaprom Y (2017) Potential improvement of biogas production from fallen teak leaves with co-digestion of microalgae. 3. Biotech 8:123
Chuanchai A, Ramaraj R (2018) Sustainability assessment of biogas production from buffalo grass and dung: biogas purification and biofertilizer. 3. Biotech 8(3):151
Demirbas A (2011) Competitive liquid biofuels from biomass. Appl Energy 88:17–28
Unpaprom Y, Intasaen O, Yongphet P, Ramaraj R (2015) Cultivation of microalga Botryococcus braunii using red Nile tilapia effluent medium for biogas production. J Ecol Environ Sci 3(2):58–65
Manmai M, Bautista K, Unpaprom Y, Ramaraj R (2019) Optimization of combined pre-treatments on sugarcane leaves for bioethanol production. Maejo Int J Energ Environ Comm 1(1):30–39
Ramaraj R, Dussadee N (2015) Biological purification processes for biogas using algae cultures: a review. J Renew Sustain Energy 4:20–32
Nelson DL, Cox MM (2008) Chapter 20 carbohydrate biosynthesis in plants and bacteria. Principles of Biochemistry The edition 5th: 751–786
Kaewdiew J, Ramaraj R, Koonaphapdeelert S, Dussadee N (2019) Assessment of the biogas potential from agricultural waste in northern Thailand. Maejo Int J Energ Environ Comm 1(1):40–47
Dussadee N, Ramaraj R, Cheunbarn T (2017) Biotechnological application of sustainable biogas production through dry anaerobic digestion of Napier grass. 3. Biotech 7:47
Vu PT, Unpaprom Y, Ramaraj R (2017) Evaluation of bioethanol production from rice field weed biomass. Emer Life Sci Res 3(2):42–49
Pantawong R, Chuanchai A, Thipbunrat P, Unpaprom Y, Ramaraj R (2015) Experimental investigation of biogas production from water lettuce, Pistia stratiotes L. Emer Life Sci Res 1(2):41–46
Dussadee N, Reansuwan K, Ramaraj R (2014) Potential development of compressed bio-methane gas production from pig farms and elephant grass silage for transportation in Thailand. Bioresour Technol 155:438–441
Tran GV, Unpaprom Y, Ramaraj R (2019) Methane productivity evaluation of an invasive wetland plant, common reed. Biomass Convers Bior. https://doi.org/10.1007/s13399-019-00451-z
Castañeda REQ, Mallol JLF (2013) Hydrolysis of biomass mediated by cellulases for the production of sugars. Sustainable Degradation of Lignocellulosic Biomass 119–155
Akhtar N, Gupta K, Goyal D, Goyal A (2016) Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy 35:489–511
Dahadha S, Amin Z, Bazyar Lakeh AA, Elbeshbishy E (2017) Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuel 31:10335–10347
Kumar R, Sharma RK, Singh AP (2017) Cellulose based grafted biosorbents-journey from lignocellulose biomass to toxic metal ions sorption applications-a review. J Mol Liq 232:62–93
Xu C, Ferdosian F (2017) Conversion of lignin into bio-based chemicals and materials. Springer Heidelberg, Berlin
Zhou S, Raouche S, Grisel S, Sigoillot J, Herpoël-Gimbert I (2017) Efficient biomass pretreatment using the white-rot fungus polyporus brumalis. Fungal Genom Biol 7:2
Baruah J, Nath BK, Sharma R, Kumar S, Deka RC, Baruah DC, Kalita E (2018) Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front Energy Res 6(141)
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58
Maurya DP, Singla A, Negi S (2015) An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3. Biotech 5:597–609
Casabar JY, Unpaprom Y, Ramaraj R (2019) Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Convers Bior 9:761–765
Preshanthan M, Kana EBG (2015) Optimization of xylose and glucose production from sugarcane leaves (Saccharum officinarum) using hybrid pretreatment techniques and assessment for hydrogen generation at semi pilot scale. Int J Hydrog Energy 40:3859–3867
Ramaraj R, Unpaprom Y (2019) Optimization of pretreatment condition for ethanol production from Cyperus difformis by response surface methodology. 3. Biotech 9(6):218
Box GEP, Cox RD (1951) On the experimental attainment of optimum conditions. J R Stat Soc Ser B Methodol 13(1):1–45
Ghelich R, Jahannama MR, Abdizadeh H, Torknik FS, Vaezi MR (2019) Central composite design (CCD)-response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Compos B Eng 166:527–541
Miller GAIL (1959) Use of dinitrosalicylic acid reagent for detection of reducing sugars. Anal Chem 31(3):426–428
Vu PT, Unpaprom Y, Ramaraj R (2018) Impact and significance of alkaline-oxidant pretreatment on the enzymatic digestibility of Sphenoclea zeylanica for bioethanol production. Bioresour Technol 247:125–130
Ramaraj R, Unpaprom Y (2019) Enzymatic hydrolysis of small-flowered nutsedge (Cyperus difformis) with alkaline pretreatment for bioethanol production. Maejo Int J Sci Technol 13(02):110–120
Bautista B, Unpaprom Y, Ramaraj R (2018) Bioethanol production from corn stalk juice using Saccharomyces cerevisiae TISTR 5020. Energ Source, Part A 41(13):1–7
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Thangavelu SK, Ahmed AS, Ani FN (2014) Bioethanol production from sago pith waste using microwave hydrothermal hydrolysis accelerated by carbon dioxide. Appl Energy 128:277–283
Hesami SM, Zilouei H, Karimi K, Asadinezhad A (2015) Enhanced biogas production from sunflower stalks using hydrothermal and organosolv pretreatment. Ind Crop Prod 76:449–455
Zheng Y, Lee C, Yu C, Cheng YS, Zhang R, Jenkins BM, VanderGheynst JS (2013) Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl Energy 1(105):1–7
Sharma SK, Kalra KL, Grewal HS (2002 Oct 1) Fermentation of enzymatically saccharified sunflower stalks for ethanol production and its scale up. Bioresour Technol 85(1):31–33
Vaithanomsat P, Chuichulcherm S, Apiwatanapiwat W (2009) Bioethanol production from enzymatically saccharified sunflower stalks using steam explosion as pretreatment. InProceedings of World Academy of Science, Engineering and Technology 37:140–143
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manmai, N., Unpaprom, Y. & Ramaraj, R. Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Conv. Bioref. 11, 1759–1773 (2021). https://doi.org/10.1007/s13399-020-00602-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00602-7