Abstract
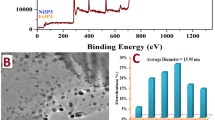
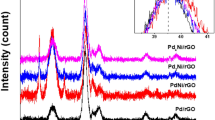
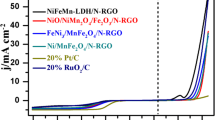
This study was conducted to exploit the properties of nitrogen-doped reduced graphene oxide (N-rGO) as support material for formic acid fuel cell. Nitrogen-doped reduced graphene oxide was synthesized by the hydrothermal synthesis method using graphene oxide (GO) flakes and urea as a nitrogen source. Palladium and iron with controllable atomic ratio were used as the active metals. Graphene oxide and carbon nanotube-supported PdFe nanoparticles were synthesized for comparison. The structure, morphology, and chemical composition of the synthesized catalysts were ascertained by X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS). The average particle sizes for Pd3Fe/N-rGO and Pd/N-rGO were 4.65 and 3.95 nm, respectively. The electrochemical characterizations (CO stripping, cyclic voltammetry, and chronoamperometry) showed that the Pd3Fe/N-rGO electrocatalyst had higher electrocatalytic activity and stability compared with that of Pd3Fe/rGO and Pd3Fe/CNT. The mass activity of Pd3Fe/N-rGO in 0.5 M of HCOOH and 0.5 M of H2SO4 solutions was 1463.9 mAmg−1 Pd, which was 3.3 and 1.35 times that of the activity obtained with graphene oxide and carbon nanotubes with the same composition, respectively. The superior performance of the Pd3Fe/N-rGO catalyst was ascribed to the presence of nitrogen functionalities in the nitrogen-doped reduced GO and the synergistic interaction between Pd and Fe nanoparticles. Nitrogen-doped reduced GO promoted the formation of smaller and narrowly distributed nanoparticles and exerted favorable electronic effects because of electron transfer from N to Pd. Therefore, Pd3Fe/N-rGO can serve as a potential electrocatalyst for the oxidation of formic acid.








Similar content being viewed by others
References
Yan, W.; Xiang, Y.; Zhang, J.; Lu, S.; Jiang, S.P.: Substantially enhanced power output and durability of direct formic acid fuel cells at elevated temperatures. Adv. Sustain. Syst. 4, 2000065 (2020)
Eppinger, J.; Huang, K.-W.: Formic acid as a hydrogen energy carrier. ACS Energy Lett. 2, 188–195 (2017)
Hossain, S.S.; Saleem, J.; Rahman, S.; Zaidi, S.M.J.; McKay, G.; Cheng, C.K.: Synthesis and evaluation of copper-supported titanium oxide nanotubes as electrocatalyst for the electrochemical reduction of carbon oxide to organics. Catalysts 9, 298 (2019)
Cuesta, A.; Cabello, G.; Osawa, M.; Gutiérrez, C.: Mechanism of the electrocatalytic oxidation of formic acid on metals. ACS Catal. 2, 728–738 (2012)
Wang, Y.; Qi, Y.; Zhang, D.: New mechanism of the direct pathway for formic acid oxidation on Pd(111). Comput. Theoret. Chem. 1049, 51–54 (2014)
Choi, S.-I.; Herron, J.A.; Scaranto, J.; Huang, H.; Wang, Y.; Xia, X.; et al.: A comprehensive study of formic acid oxidation on palladium nanocrystals with different types of facets and twin defects. Chem. Cat. Chem. 7, 2077–2084 (2015)
Ferre-Vilaplana, A.; Perales-Rondón, J.V.; Buso-Rogero, C.; Feliu, J.M.; Herrero, E.: Formic acid oxidation on platinum electrodes: a detailed mechanism supported by experiments and calculations on well-defined surfaces. J. Mater. Chem. A. 5, 21773–21784 (2017)
Yousaf, A.B.; Imran, M.; Zeb, A.; Xie, X.; Liang, K.; Zhou, X.; et al.: Synergistic effect of graphene and multi-walled carbon nanotubes composite supported Pd nanocubes on enhancing catalytic activity for electro-oxidation of formic acid. Catal. Sci. Technol. 6, 4794–4801 (2016)
Hu, S.; Scudiero, L.; Ha, S.: Electronic effect of Pd-transition metal bimetallic surfaces toward formic acid electrochemical oxidation. Electrochem. Commun. 38, 107–109 (2014)
Matin, M.A.; Jang, J.-H.; Kwon, Y.-U.: PdM nanoparticles (M = Ni Co, Fe, Mn) with high activity and stability in formic acid oxidation synthesized by sonochemical reactions. J. Power Sourc. 262, 356–363 (2014)
Ma, Y.; Li, T.; Chen, H.; Chen, X.; Deng, S.; Xu, L.; et al.: A general strategy to the synthesis of carbon-supported PdM (M = Co, Fe and Ni) nanodendrites as high-performance electrocatalysts for formic acid oxidation. J. Energy Chem. 26, 1238–1244 (2017)
Juárez-Marmolejo, L.; Pérez-Rodríguez, S.; Montes de Oca-Yemha, M.G.; Palomar-Pardavé, M.; Romero-Romo, M.; Ezeta-Mejía, A.; et al.: Carbon supported PdM (M = Fe, Co) electrocatalysts for formic acid oxidation. Influence of the Fe and Co precursors. Int. J. Hydrogen Energy 44, 1640–1649 (2019)
Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H.: Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J.Hydrogen Energy 44, 14744–14769 (2019)
Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Mikolajczuk-Zychora, A.; Mierzwa, B.; Borodzinski, A.; Stobinski, L.: A simple method for enhancing the catalytic activity of Pd deposited on carbon nanotubes used in direct formic acid fuel cells. Appl. Surf. Sci. 476, 806–814 (2019)
Zhou, Y.; Zhu, X.; Zhang, B.; Ye, D.-D.; Chen, R.; Liao, Q.: High performance formic acid fuel cell benefits from Pd–PdO catalyst supported by ordered mesoporous carbon. Int. J. Hydrogen Energy 45, 29235–29245 (2020)
Çögenli, M.S.; Bayrakçeken, Yurtcan A.: Heteroatom doped 3D graphene aerogel supported catalysts for formic acid and methanol oxidation. Int. J. Hydrogen Energy 45, 650–666 (2020)
Iqbal, M.Z.; Rehman, A.-U.; Siddique, S.: Prospects and challenges of graphene based fuel cells. J. Energy Chem. 39, 217–234 (2019)
Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; et al.: Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 39, 45–65 (2020)
Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y.: Heteroatom-doped carbon materials: synthesis, mechanism, and application for sodium-ion batteries. Small Methods 3, 1800323 (2019)
Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S.: A review of nitrogen-doped graphene catalysts for proton exchange membrane fuel cells-synthesis, characterization, and improvement. Nano-Struct. Nano-Obj. 15, 140–152 (2018)
Kaur, M.; Kaur, M.; Sharma, V.K.: Nitrogen-doped graphene and graphene quantum dots: a review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 259, 44–64 (2018)
Jin, Y.; Zhao, J.; Li, F.; Jia, W.; Liang, D.; Chen, H.; et al.: Nitrogen-doped graphene supported palladium-nickel nanoparticles with enhanced catalytic performance for formic acid oxidation. Electrochim. Acta 220, 83–90 (2016)
Chowdhury, S.R.; Maiyalagan, T.: Enhanced electro-catalytic activity of nitrogen-doped reduced graphene oxide supported PdCu nanoparticles for formic acid electro-oxidation. Int. J. Hydrogen Energy 44, 14808–14819 (2019)
Shao, Y.; Sui, J.; Yin, G.; Gao, Y.: Nitrogen-doped carbon nanostructures and their composites as catalytic materials for proton exchange membrane fuel cell. Appl. Catal. B Environ. 79, 89–99 (2008)
Xu, H.; Yan, B.; Li, S.; Wang, J.; Wang, C.; Guo, J.; et al.: N-doped graphene supported PtAu/Pt intermetallic core/dendritic shell nanocrystals for efficient electrocatalytic oxidation of formic acid. Chem. Eng. J. 334, 2638–2646 (2018)
Sun, Y.; Zhou, T.; Pan, Q.; Zhang, X.; Guo, J.: PtFe/nitrogen-doped graphene for high-performance electrooxidation of formic acid with composition sensitive electrocatalytic activity. RSC Adv. 5, 60237–60245 (2015)
Li, W.; Zhou, T.; Le, Z.; Liao, M.; Liu, H.; Na, B.; et al.: Effect of thermal treatment of Pd decorated Fe/C nanocatalysts on their catalytic performance for formic acid oxidation. RSC Adv. 8, 35496–35502 (2018)
Malolepszy, A.; Mazurkiewicz, M.; Stobinski, L.; Lesiak, B.; Kövér, L.; Tóth, J.; et al.: Deactivation resistant Pd–ZrO2 supported on multiwall carbon nanotubes catalyst for direct formic acid fuel cells. Int. J. Hydrogen Energy 40, 16724–16733 (2015)
Man, R.W.Y.; Brown, A.R.C.; Wolf, M.O.: Mechanism of formation of palladium nanoparticles: lewis base assisted, low-temperature preparation of monodisperse nanoparticles. Angew. Chem. Int. Ed. 51, 11350–11353 (2012)
Liao, M.; Hu, Q.; Zheng, J.; Li, Y.; Zhou, H.; Zhong, C.-J.; et al.: Pd decorated Fe/C nanocatalyst for formic acid electrooxidation. Electrochim. Acta 111, 504–509 (2013)
Cai, B.; Ma, Y.; Wang, S.; Yi, N.; Zheng, Y.; Qiu, X.; et al.: Facile synthesis of PdFe alloy tetrahedrons for boosting electrocatalytic property towards formic acid oxidation. Nanoscale 11, 18015–18020 (2019)
Aslam, Z.; Qaiser, M.; Ali, R.; Abbas, A.; Ihsanullah, F.; Zarin, S.: Al2O3/MnO2/CNTs nanocomposite: synthesis, characterization and phenol adsorption. Fullerenes, Nanotubes, Carbon Nanostruct. 27, 591–600 (2019)
Zarin, S.; Aslam, Z.; Zahir, A.; Kamal, M.S.; Rana, A.G.; Ahmad, W.; et al.: Synthesis of bimetallic/carbon nanocomposite and its application for phenol removal. J. Iran. Chem. Soc. 15, 2689–2701 (2018)
Akhtar, A.; Aslam, Z.; Asghar, A.; Bello, M.M.; Raman, A.A.A.: Electrocoagulation of Congo Red dye-containing wastewater: optimization of operational parameters and process mechanism. J. Environ. Chem. Eng. 8, 104055 (2020)
Zhou, W.; Lee, J.Y.: Particle size effects in Pd-Catalyzed electrooxidation of formic acid. J. Phys. Chem. C 112, 3789–3793 (2008)
Zhan, D.; Velmurugan, J.; Mirkin, M.V.: Adsorption/Desorption of hydrogen on Pt nanoelectrodes: evidence of surface diffusion and spillover. J. Am. Chem. Soc. 131, 14756–14760 (2009)
Zhang, X.; Yin, H.; Wang, J.; Chang, L.; Gao, Y.; Liu, W.; et al.: Shape-dependent electrocatalytic activity of monodispersed palladium nanocrystals toward formic acid oxidation. Nanoscale 5, 8392–8397 (2013)
Han, B.; Xu, C.: Nanoporous PdFe alloy as highly active and durable electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 39, 18247–18255 (2014)
Xing, Z.; Guo, Z.; Chen, X.; Zhang, P.; Yang, W.: Optimizing the activity of Pd based catalysts towards room-temperature formic acid decomposition by Au alloying. Catal. Sci. Technol. 9, 588–592 (2019)
Ulas, B.; Caglar, A.; Kivrak, H.: Determination of optimum Pd: Ni ratio for PdxNi100-x/CNTs formic acid electrooxidation catalysts synthesized via sodium borohydride reduction method. Int. J. Energy Res. 43, 3436–3445 (2019)
Yuan, D.; Zhang, Y.: Theoretical investigations of HCOOH decomposition on ordered Cu-Pd alloy surfaces. Appl. Surf. Sci. 462, 649–658 (2018)
Wang, H.; Li, Y.; Li, C.; Wang, Z.; Xu, Y.; Li, X.; et al.: Hyperbranched PdRu nanospine assemblies: an efficient electrocatalyst for formic acid oxidation. J. Mater. Chem. A. 6, 17514–17518 (2018)
Kiyani, R.; Rowshanzamir, S.; Parnian, M.J.: Nitrogen doped graphene supported palladium-cobalt as a promising catalyst for methanol oxidation reaction: synthesis, characterization and electrocatalytic performance. Energy 113, 1162–1173 (2016)
She, Y.; Lu, Z.; Fan, W.; Jewell, S.; Leung, M.K.H.: Facile preparation of PdNi/rGO and its electrocatalytic performance towards formic acid oxidation. J. Mater. Chem. A. 2, 3894–3898 (2014)
Mikołajczuk, A.; Borodzinski, A.; Kedzierzawski, P.; Stobinski, L.; Mierzwa, B.; Dziura, R.: Deactivation of carbon supported palladium catalyst in direct formic acid fuel cell. Appl. Surf. Sci. 257, 8211–8214 (2011)
Yu, X.; Pickup, P.G.: Mechanistic study of the deactivation of carbon supported Pd during formic acid oxidation. Electrochem. Commun. 11, 2012–2014 (2009)
Xiong, B.; Zhou, Y.; Zhao, Y.; Wang, J.; Chen, X.; O’Hayre, R.; et al.: The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 52, 181–192 (2013)
Ma, Jh; Wang, L.; Mu, X.; Li, L.: Nitrogen-doped graphene supported Pt nanoparticles with enhanced performance for methanol oxidation. Int. J. Hydrogen Energy 40, 2641–2647 (2015)
Xu, H.; Yan, B.; Zhang, K.; Wang, J.; Li, S.; Wang, C.; et al.: N-doped graphene-supported binary PdBi networks for formic acid oxidation. Appl. Surf. Sci. 416, 191–199 (2017)
Zhang, X.; Zhu, J.; Tiwary, C.S.; Ma, Z.; Huang, H.; Zhang, J.; et al.: Palladium nanoparticles supported on nitrogen and sulfur dual-doped graphene as highly active electrocatalysts for formic acid and methanol oxidation. ACS Appl. Mater. Interfaces. 8, 10858–10865 (2016)
Zhu, J.; Xiao, M.; Zhao, X.; Li, K.; Liu, C.; Xing, W.: Nitrogen-doped carbon–graphene composites enhance the electrocatalytic performance of the supported Pt catalysts for methanol oxidation. Chem. Commun. 50, 12201–12203 (2014)
Hu, S.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S.: Carbon supported Pd-based bimetallic and trimetallic catalyst for formic acid electrochemical oxidation. Appl. Catal. B Environ. 180, 758–765 (2016)
Zhang, Z.; Gong, Y.; Wu, D.; Li, Z.; Li, Q.; Zheng, L.; et al.: Facile fabrication of stable PdCu clusters uniformly decorated on graphene as an efficient electrocatalyst for formic acid oxidation. Int. J. Hydrogen Energy 44, 2731–2740 (2019)
Chen, D.; Sun, P.; Liu, H.; Yang, J.: Bimetallic Cu–Pd alloy multipods and their highly electrocatalytic performance for formic acid oxidation and oxygen reduction. J. Mater. Chem. A. 5, 4421–4429 (2017)
Acknowledgements
This work was supported by the Deanship of Scientific Research at King Faisal University via annual research grant (no. 150212). The author would also like to thank Dr. Said Mansur, Qatar Environment & Energy Research Institute, Hamad Bin Khalifa University, for helping in characterizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Hossain, S.S. Bimetallic Pd–Fe Supported on Nitrogen-Doped Reduced Graphene Oxide as Electrocatalyst for Formic Acid Oxidation. Arab J Sci Eng 46, 6543–6556 (2021). https://doi.org/10.1007/s13369-020-05192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-05192-0