Abstract
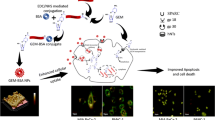
The current advent explores the potential of itraconazole (ITR) in prostate cancer (PCa), by its incorporation into albumin nanoparticles (NP). ITR as a repurposed moiety has displayed tremendous potential in various cancers. However, poor aqueous solubility poses hurdles towards its clinical translation. Amorphisation of ITR was observed post-incorporation within NP matrix which could prevent its precipitation in aqueous media. ITR NP was developed using quality by design and multivariate analysis and evaluated for cellular uptake, cell proliferation inhibition and the mechanism of PCa cell inhibition. Time and concentration-dependent serum stability and hemolytic potential revealed safety of ITR NP. Morphological changes and nuclear staining studies revealed the efficacy of ITR and ITR NP in promoting growth inhibition of PC-3 cells. Superior qualitative and quantitative uptake, reactive oxygen species (ROS) and mitochondrial impairment for ITR NP in comparison with ITR and control group was observed. Cell cycle study revealed remarkable G2/M phase inhibition in PC-3 cells. ITR NP demonstrated superior anticancer potential in 3D tumoroids mimicking the micro-metastatic lesions compared to control and ITR. Hence, ITR NP can be a favorable alternative therapeutic alternative in PCa.
Graphical Abstract











Similar content being viewed by others
Availability of data and materials
The datasets generated will be made available on reasonable request.
References
Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: Global status and temporal trends in 89 countries from 2000 to 2019. Front Public Heal. 2022;10.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Kichloo A, Albosta M, Dahiya D, Guidi JC, Aljadah M, Singh J, et al. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J Clin Oncol. 2021;12:150–63.
Lee M, Hong H, Kim W, Zhang L, Friedlander TW, Fong L, et al. Itraconazole as a noncastrating treatment for biochemically recurrent prostate cancer: A phase 2 study. Clin Genitourin Cancer. 2019;17:e92–6.
Li CL, Fang ZX, Wu Z, Hou YY, Wu HT, Liu J. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed Pharmacother. 2022;154.
Hardin TC, Graybill JR, Fetchick R, Woestenborghs R, Rinaldi MG, Kuhn JG. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988;32:1310–3.
Prentice AG, Glasmacher A. Making sense of itraconazole pharmacokinetics. J Antimicrob Chemother. 2005;56.
Famta P, Shah S, Vambhurkar G, Srinivasarao DA, Jain N, Begum N, et al. Quality by design endorsed fabrication of Ibrutinib-loaded human serum albumin nanoparticles for the management of leukemia. Eur J Pharm Biopharm. 2023;190:94–106.
Adick A, Hoheisel W, Schneid S, Mulac D, Azhdari S, Langer K. Challenges of nanoparticle albumin bound (nab™) technology: Comparative study of Abraxane® with a newly developed albumin-stabilized itraconazole nanosuspension. Eur J Pharm Biopharm. 2023;193:129–43.
Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99–105.
Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–8.
Zhang L, Liu Z, Yang K, Kong C, Liu C, Chen H, et al. Tumor progression of non-small cell lung cancer controlled by albumin and micellar nanoparticles of itraconazole, a multitarget angiogenesis inhibitor. Mol Pharm. 2017;14:4705–13.
Jin G, Jin M, Yin X, Jin Z, Chen L, Gao Z. A comparative study on the effect of docetaxel-albumin nanoparticles and docetaxel-loaded PEG-albumin nanoparticles against non-small cell lung cancer. Int J Oncol. 2015;47:1945–53.
Zhang S, Asghar S, Yang L, Hu Z, Chen Z, Shao F, et al. Borneol and poly (ethylene glycol) dual modified BSA nanoparticles as an itraconazole vehicle for brain targeting. Int J Pharm. 2020;575.
Zhao D, Zhao X, Zu Y, Li J, Zhang Y, Jiang R, et al. Preparation, characterization, and in vitro targeted delivery of folate-decorated paclitaxel-loaded bovine serum albumin nanoparticles. Int J Nanomedicine. 2010;5:669–77.
Famta P, Shah S, Jain N, Srinivasarao DA, Murthy A, Ahmed T, et al. Albumin-hitchhiking: Fostering the pharmacokinetics and anticancer therapeutics. J Control Release. 2023;353:166–85.
Mourya A, Famta P, Shah S, Srinivasarao DA, Sharma A, Vambhurkar G, et al. Raloxifene loaded D-α-tocopherol polyethylene glycol 1000 succinate stabilized poly (ε-caprolactone) nanoparticles augmented drug delivery and apoptosis in breast cancer cells. J Drug Deliv Sci Technol. 2024;92.
Boateng-Marfo Y, Dong Y, Loh ZH, Lin H, Ng WK. Intravenous human serum albumin (HSA)-bound artemether nanoparticles for treatment of severe malaria. Colloids Surf A Physicochem Eng Asp. 2018;536:20–9.
Charankumar K, Bagasariya D, Jain N, Famta P, Shah S, Vambhurkar G, et al. Quality by design (QbD) abetted development of pioglitazone incorporated liposomes-loaded hyaluronic acid-based in situ hydrogel for the management of melanoma. J Drug Deliv Sci Technol. 2023;84:104453.
Kumar R, Singh A, Sharma K, Dhasmana D, Garg N, Siril PF. Preparation, characterization and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles. Mater Sci Eng C. 2020;106.
Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6:399–408.
Yang Y, Huang Z, Li J, Mo Z, Huang Y, Ma C, et al. PLGA porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: Novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv Healthc Mater. 2019;8.
Kommineni N, Mahira S, Domb AJ, Khan W. Cabazitaxel-loaded nanocarriers for cancer therapy with reduced side effects. Pharmaceutics. 2019;11:1–22.
Shah S, Famta P, Fernandes V, Bagasariya D, Charankumar K, Kumar Khatri D, et al. Quality by design steered development of niclosamide loaded liposomal thermogel for melanoma: in vitro and ex vivo evaluation. Eur J Pharm Biopharm. 2022;180:119–36.
Wang L, Liu Y, Li W, Jiang X, Ji Y, Wu X, et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: Implications for cancer therapy. Nano Lett. 2011;11:772–80.
Sivandzade F, Bhalerao A, Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio-protocol. 2019;9.
Pioch J, Blomgran R. Optimized flow cytometry protocol for dihydrorhodamine 123-based detection of reactive oxygen species in leukocyte subpopulations in whole blood. J Immunol Methods. 2022;507.
Shah S, Famta P, Kumar R, Sharma A, Vambhurkar G, Pandey G, et al. Quality by design fostered fabrication of cabazitaxel loaded pH-responsive Improved nanotherapeutics against prostate cancer. Colloids Surf B Biointerfaces. 2024;234:113732.
Sonam, Chaudhary H, Kumar V. Taguchi design for optimization and development of antibacterial drug-loaded PLGA nanoparticles. Int J Biol Macromol. 2014;64:99–105.
Rampado R, Peer D. Design of experiments in the optimization of nanoparticle-based drug delivery systems. J Control Release. 2023;358:398–419.
Ngan CL, Basri M, Lye FF, Fard Masoumi HR, Tripathy M, Abedi Karjiban R, et al. Comparison of Box-Behnken and central composite designs in optimization of fullerene loaded palm-based nano-emulsions for cosmeceutical application. Ind Crops Prod. 2014;59:309–17.
Kasekar NM, Singh S, Jadhav KR, Kadam VJ. BCS class II drug loaded protein nanoparticles with enhanced oral bioavailability: in vitro evaluation and in vivo pharmacokinetic study in rats. Drug Dev Ind Pharm. 2020;46:955–62.
Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MNV. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85.
Shah S, Rangaraj N, Singh SB, Srivastava S. Exploring the unexplored avenues of surface charge in nano-medicine. Colloids Interface Sci Commun. Elsevier; 2021;42:100406.
Rangaraj N, Pailla SR, Shah S, Prajapati S, Sampathi S. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Deliv Transl Res. 2020;10:1476–94.
Ho HN, Tran TH, Tran TB, Yong CS, Nguyen CN. Optimization and characterization of artesunate-loaded chitosan-decorated poly(D,L-lactide-co-glycolide) acid nanoparticles. J Nanomater. 2015;2015.
da Silva Júnior WF, de Oliveira Pinheiro JG, Moreira CDLFA, de Souza FJJ, de Lima ÁAN. Alternative technologies to improve solubility and stability of poorly water-soluble Drugs. Multifunct Syst Comb Deliv Biosensing Diagnostics. 2017;281–305.
Kaboli SF, Mehrnejad F, Nematollahzadeh A. Molecular modeling prediction of albumin-based nanoparticles and experimental preparation, characterization, and in-vitro release kinetics of prednisolone from the nanoparticles. J Drug Deliv Sci Technol. 2021;64:102588.
Alshetaili AS, Ansari MJ, Anwer MK, Ganaie MA, Iqbal M, Alshahrani SM, et al. Enhanced oral bioavailability of ibrutinib encapsulated poly (Lactic-co- Glycolic Acid) nanoparticles: Pharmacokinetic evaluation in rats. Curr Pharm Anal. 2019;15:661–8.
Jana B, Kim S, Chae JB, Chung H, Kim C, Ryu JH. Mitochondrial membrane disrupting molecules for selective killing of senescent cells. ChemBioChem. 2021;22:3391–7.
Vinarov Z, Gancheva G, Burdzhiev N, Tcholakova S. Solubilization of itraconazole by surfactants and phospholipid-surfactant mixtures: interplay of amphiphile structure, pH and electrostatic interactions. J Drug Deliv Sci Technol. 2020;57:101688.
Xu C, Zhuo Y, Liu Y, Chen H. Itraconazole inhibits the growth of cutaneous squamous cell carcinoma by targeting HMGCS1/ACSL4 axis. Front Pharmacol. 2022;13:828983.
John R, Dalal B, Shankarkumar A, Devarajan PV. Innovative Betulin Nanosuspension exhibits enhanced anticancer activity in a Triple Negative Breast Cancer Cell line and Zebrafish angiogenesis model. Int J Pharm. 2021;600.
Acknowledgements
The authors acknowledge the research funding support by the Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Govt. of India to “Pharmaceutical Innovation and Translational Research Lab” (PITRL), Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER) Hyderabad, INDIA.
Funding
None.
Author information
Authors and Affiliations
Contributions
Saurabh Shah—Conceptualization, Methodology, experimentation, software validation, Original draft writing; Paras Famta – Methodology, experimentation, software validation, Original draft writing; Anamika Sharma, Rahul Kumar, Giriraj Pandey, Ganesh Vambhurkar – Data curation, formal analysis; Dadi A. Srinivasarao, Akshay Shinde, Sajja Bhanu Prasad – Manuscript review editing; Amit Asthana—Supervision, and Saurabh Srivastava – Conceptualization, Supervision, Validation.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors give consent to publication.
Competing interests
The authors declare no relevant financial or non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S., Famta, P., Sharma, A. et al. Quality by design empowered preparation of itraconazole albumin nanoparticles for prostate cancer. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01592-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-024-01592-z