Abstract
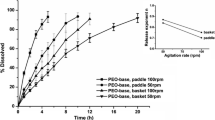
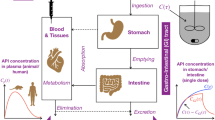
Standard dissolution testing methods typically do not correlate strongly with the in vivo drug release behavior for the oral delivery products, since they only focus on the drug dissolution in the gastric/intestinal fluid and do not account for the intestinal absorption of drug. Artificial gastrointestinal systems attempt to bridge this gap by using dialysis membranes as a proxy for the intestinal membranes. We present a systematic proof-of-concept study of how the drug dissolution and drug absorption are mimicked in such systems for the case of polymer-drug formulations. We utilize a modified version of the conventional shaking-flask test, in which the drug formulation is placed inside a dialysis bag. Dissolution experiments are performed on a commercial aspirin formulation and model formulations of aspirin with varying amounts of poly-methyl-methacrylate-co-methacrylic acid (PMA-MAA), both for conventional and modified shaking-flask test. Results are successfully interpreted using a simple model that assumes first-order kinetics for both the drug release from the formulation and drug permeation through the membrane. The differences between the model and commercial formulations and the effects of shaking speed and drug loading are established by comparison of the first-order rate constants. Finally, comparison with a reported in vivo study demonstrates how the modified shaking-flask setup can be used to improve the in vitro in vivo correlation.

Graphical abstract






Similar content being viewed by others
References
Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2012;64:327–41. https://doi.org/10.1016/j.addr.2012.09.014.
Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for drug delivery systems. Annu Rev Chem Biomol 2010;1:149–73. https://doi.org/10.1146/annurev-chembioeng-073009-100847.Polymers.
Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–98. https://doi.org/10.1021/cr940351u.
Schmaljohann D. Thermo- and PH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58(15):1655–70. https://doi.org/10.1016/j.addr.2006.09.020.
Marques MRC; Loebenberg R; Almukainzi M Simulated biologic fluids with possible application in dissolution testing. Dissolution Technol. 2011, No. August, 15–28. https://doi.org/10.1002/jps.23029.
Abouelmagd SA, Sun B, Chang AC, Ku YJ, Yeo Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: are we doing it right? Mol Pharmaceut 2015;12(3):997–1003. https://doi.org/10.1021/mp500817h.
Scholz A, Kostewicz E, Abrahamsson B, Dressman JB. Can the USP paddle method be used to represent in-vivo hydrodynamics? J Pharm Pharmacol. 2003;55(4):443–51. https://doi.org/10.1211/002235702946.
Amidon GL; Sinko PJ; Fleisher D Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res 1988, pp 651–654. https://doi.org/10.1023/A:1015927004752.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral-drug delivery systems - prediction of intestinal-absorption. Adv Drug Deliv Rev. 1996;19:359–76.
Hens B, Corsetti M, Spiller R, Marciani L, Vanuytsel T, Tack J, et al. Exploring gastrointestinal variables affecting drug and formulation behavior: methodologies, challenges and opportunities. Int J Pharm. 2017;519(1–2):79–97. https://doi.org/10.1016/j.ijpharm.2016.11.063.
Shargel L; Wu-Pong S; Yu ABC Applied Biopharmaceutics & Pharmacokinetics; Appleton & Lange Reviews/McGraw-Hill, Medical Pub. Division, 2005.
Yu LX, Crison JR, Amidon GL. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int J Pharm. 1996;140(1):111–8. https://doi.org/10.1016/0378-5173(96)04592-9.
Willmann S, Schmitt W, Keldenich J, Dressman JB. A physiologic model for simulating gastrointestinal flow and drug absorption in rats. Pharm Res. 2003;20(11):1766–71. https://doi.org/10.1023/B:PHAM.0000003373.72652.c0.
Abbiati RA, Lamberti G, Grassi M, Trotta F, Manca D. Definition and validation of a patient-individualized physiologically-based pharmacokinetic model. Comput Chem Eng. 2016;84:394–408. https://doi.org/10.1016/j.compchemeng.2015.09.018.
Cascone S, Lamberti G, Piazza O, Abbiati RA, Manca D. A physiologically-based model to predict individual pharmacokinetics and pharmacodynamics of remifentanil. Eur J Pharm Sci. 2018;111:20–8. https://doi.org/10.1016/j.ejps.2017.09.028.
Grassi M, Lamberti G, Cascone S, Grassi G. Mathematical modeling of simultaneous drug release and in vivo absorption. Int J Pharm. 2011;418(1):130–41. https://doi.org/10.1016/j.ijpharm.2010.12.044.
Ferrua MJ, Singh RP. Modeling the fluid dynamics in a human stomach to gain insight of food digestion. J Food Sci. 2010;75(7):151–62. https://doi.org/10.1111/j.1750-3841.2010.01748.x.
Levy G, Leonards JR, Procknal JA. Development of in vitro dissolution tests which correlate quantitatively with dissolution rate-limited drug absorption in man. J Pharm Sci. 1965;54(12):1719–22. https://doi.org/10.1002/jps.2600541204.
Blanquet S, Zeijdner E, Beyssac E, Meunier JP, Denis S, Havenaar R, et al. A dynamic arificial gastrointestinal system for studying the behavior of orally administrated drug dosage forms under various physiological conditions. Pharm Res. 2004;21(4):585–91. https://doi.org/10.1023/B:PHAM.0000022404.70478.4b.
Todaro V, Persoons T, Grove G, Healy AM, D’Arcy DM. Characterization and simulation of hydrodynamics in the paddle, basket and flow-through dissolution testing apparatuses-a review. Dissolut Technol 2017;24(3):24–36. https://doi.org/10.14227/DT240317P24.
Lu Z, Fassihi R. Mechanistic approach to understanding the influence of USP apparatus I and II on dissolution kinetics of tablets with different operating release mechanisms. AAPS PharmSciTech. 2017;18(2):462–72. https://doi.org/10.1208/s12249-016-0535-x.
Arroyo E, Luque PA, Cosio M, Soto C, Villarreal R, Nava O, et al. Study of a controlled release polymeric system based on pluronic P123: spectroscopic characterization and theoretical model approach. J Mol Struct. 2017;1138:172–6. https://doi.org/10.1016/J.MOLSTRUC.2017.03.018.
Trasi NS, Purohit HS, Wen H, Sun DD, Taylor LS. Non-sink dissolution behavior and solubility limit of commercial tacrolimus amorphous formulations. J Pharm Sci. 2017;106(1):264–72. https://doi.org/10.1016/J.XPHS.2016.09.016.
Yoshida T, Lai TC, Kwon GS, Sako K. PH- and ion-sensitive polymers for drug delivery. Expert Opin Drug Del 2013;10(11):1497–513. https://doi.org/10.1517/17425247.2013.821978.
Higuchi BT, Elowe LN. The physics of tablet compression. J Am Pharm Assoc. 1954;43(11):685–9. https://doi.org/10.1002/jps.3030431118.
Katori N, Aoyagi N, Terao T. Estimation of agitation intensity in the GI tract in humans and dogs based on in vitro/in vivo correlation. Pharm Res. 1995;12(2):237–43. https://doi.org/10.1023/A:1016231010301.
Siepmann J, Peppas NA. Higuchi equation: derivation, applications, use and misuse. Int J Pharm 2011;418(1):6–12. https://doi.org/10.1016/j.ijpharm.2011.03.051.
Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2008;364(2):328–43. https://doi.org/10.1016/j.ijpharm.2008.09.004.
Souliman S, Beyssac E, Cardot JM, Denis S, Alric M. Investigation of the biopharmaceutical behavior of theophylline hydrophilic matrix tablets using USP methods and an artificial digestive system. Drug Dev Ind Pharm. 2007;33(4):475–83. https://doi.org/10.1080/03639040601128654.
Blanquet S, Zeijdner E, Beyssac E, Meunier J, Denis S, Havenaar R, et al. A Dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm Res. 2004;21(4):585–91.
Minekusr M, Marteaul P, Havenaarl R, Huis JHJ. A multicompartmental dynamic computer- controlled model simulating the stomach and small intestine. ATLA. 1995;23(January 1995):197–209.
Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted Gut Sac Model as a tool in pharmaceutical research: limitations and applications. J Pharm Pharmacol. 2012;64(3):326–36. https://doi.org/10.1111/j.2042-7158.2011.01391.x.
Ruan LP, Chen S, Yu BY, Zhu DN, Cordell GA, Qiu SX. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur J Med Chem. 2006;41(5):605–10. https://doi.org/10.1016/j.ejmech.2006.01.013.
Rosen A, Macheras P. Effect of protein on the absorption of phenytoin through everted gut preparations. J Pharm Pharmacol. 1985;37(3):154–8. https://doi.org/10.1111/j.2042-7158.1985.tb05031.x.
Xie L, Beyer S, Vogel V, Wacker MG, Mäntele W. Assessing the drug release from nanoparticles: overcoming the shortcomings of dialysis by using novel optical techniques and a mathematical model. Int J Pharm. 2015;488(1–2):108–19. https://doi.org/10.1016/j.ijpharm.2015.03.080.
Kopelman R. Fractal reaction kinetics. Science (80- ). 1988;241(4873):1620–6. https://doi.org/10.1126/science.241.4873.1620.
Macheras P; Iliadis A Modeling in biopharmaceutics, pharmacokinetics and pharmacodynamics; Interdisciplinary Applied Mathematics; Springer International Publishing: Cham, 2016; Vol. 30. https://doi.org/10.1007/978-3-319-27598-7.
Macheras, P.; Argyrakis, P. Gastrointestinal drug absorption: is it time to consider heterogeneity as well as homogeneity? Pharm Res. Kluwer Academic Publishers-Plenum Publishers 1997, pp 842–847. https://doi.org/10.1023/A:1012183313218.
Hoa NT, Kinget R. Design and evaluation of two-phase partition-dissolution method and its use in evaluating artemisinin tablets. J Pharm Sci. 1996;85(10):1060–3. https://doi.org/10.1021/js960115u.
Charman WN, Charman SA, Monkhouse DC, Frisbee SE, Lockhart EA, Weisman S, et al. Biopharmaceutical characterisation of a low-dose (75 Mg) controlled-release aspirin formulation. Br J Clin Pharmacol. 1993;36(5):470–3. https://doi.org/10.1111/j.1365-2125.1993.tb00399.x.
Manga RD, Jha PK. Mathematical models for controlled drug release through PH-responsive polymeric hydrogels. J Pharm Sci. 2017;106(2):629–38. https://doi.org/10.1016/j.xphs.2016.10.019.
Gupta PK, Hung CT, Perrier DG. Quantitation of the release of doxorubicin from colloidal dosage forms using dynamic dialysis. J Pharm Sci. 1987;76(2):141–5.
Schmuckler JS. Buffer Solutions. J Chem Educ. 1982;59(4):305. https://doi.org/10.1021/ed059p305.4.
Khan F, Li M, Schlindwein W. Comparison of in vitro dissolution tests for commercially available aspirin tablets. Dissolution Technol. 2013;20(1):48–58. https://doi.org/10.14227/DT200113P48.
Torrado S, Cadorniga R, Torrado JJ. Effect of drug release rate on bioavailability of different aspirin tablets. Int J Pharm 1996;133(1–2):65–70. https://doi.org/10.1016/0378-5173(95)04411-6.
Bunde A, Havlin S, Nossal R, Stanley HE, Weiss GH. On controlled diffusion-limited drug release from a leaky matrix. J Chem Phys. 1985;83(11):5909–13. https://doi.org/10.1063/1.449622.
Kosmidis K, Argyrakis P, Macheras P. Fractal kinetics in drug release from finite fractal matrices. J Chem Phys. 2003;119(12):6373–7. https://doi.org/10.1063/1.1603731.
Singh K, Satapathi S, Jha PK. “Ant-Wall” model to study drug release from excipient matrix. Phys A Stat Mech its Appl. 2019;519:98–108. https://doi.org/10.1016/J.PHYSA.2018.12.029.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983;15(1):25–35. https://doi.org/10.1016/0378-5173(83)90064-9.
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. https://doi.org/10.1016/j.addr.2011.12.009.
Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22(7):2416–26. https://doi.org/10.1096/fj.07-102723.
Pedersen BT, Østergaard J, Larsen SW, Larsen C. Characterization of the rotating dialysis cell as an in vitro model potentially useful for simulation of the pharmacokinetic fate of intra-articularly administered drugs. Eur J Pharm Sci. 2005;25(1):73–9. https://doi.org/10.1016/j.ejps.2005.01.019.
Acknowledgments
The authors thank Raminder Kaur Sekhon and Yash Pal Gangwar for some preliminary work related to this paper.
Funding sources
This work is supported by a research grant from the Science and Engineering Research Board (SERB), India (no. YSS/2015/001228).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan, A., Jha, P.K. Experiments and modeling of controlled release behavior of commercial and model polymer-drug formulations using dialysis membrane method. Drug Deliv. and Transl. Res. 10, 515–528 (2020). https://doi.org/10.1007/s13346-019-00696-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00696-1