Abstract
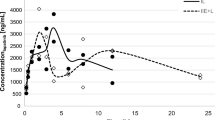
Elacridar (GF120918) is a highly potent inhibitor of both P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2), the main efflux transporters expressed at the blood-brain barrier (BBB). Elacridar shows very low aqueous solubility, which complicates its formulation for i.v. administration. An intravenous infusion protocol would be preferred to achieve high and controlled plasma concentrations of elacridar in large animals, including nonhuman primates. Formulation of elacridar for i.v. infusion was achieved using a co-solvent strategy, resulting in an aqueous dispersion with a final concentration of 5 g L−1 elacridar with tetrahydrofuran (5% w/v) in aqueous D-glucose solution (2.5%, w/v). Particle size (mean = 2.8 ± 0.9 μm) remained stable for 150 min. The preparation was i.v. administered as a continuous infusion (12 mg kg−1 h−1 for 90 min) to three baboons. Arterial and venous plasma pharmacokinetics (PK) of elacridar were monitored using a newly developed and validated HPLC-UV method. Elacridar concentration increased rapidly to reach a plateau at 9.5 μg mL−1 within 20 min after the start of infusion. Elacridar PK in venous plasma did not differ from arterial plasma facing the BBB, indicating the absence of an arteriovenous concentration gradient. Intravenous infusion of elacridar allows for controlled exposure of the BBB and offers a useful tool to assess the impact of ABCB1/ABCG2 on drug disposition to the brain in nonhuman primates, a relevant animal model for the study of transporter function at the BBB.


Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- BBB:
-
Blood-brain barrier
- CSs:
-
Calibration standards
- CNS:
-
Central nervous system
- IS:
-
Internal standard
- i.v.:
-
Intravenous
- C max :
-
Maximum plasma concentration
- PK:
-
Pharmacokinetics
- PDI:
-
Polydispersity index
- QCs:
-
Quality controls
- THF:
-
Tetrahydrofuran
- TKIs:
-
Tyrosine kinase inhibitors
References
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030.
Shawahna R, Uchida Y, Declèves X, Ohtsuki S, Yousif S, Dauchy S, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8(4):1332–41. https://doi.org/10.1021/mp200129p.
Uchida Y, Wakayama K, Ohtsuki S, Chiba M, Ohe T, Ishii Y, et al. Blood-brain barrier pharmacoproteomics-based reconstruction of the in vivo brain distribution of P-glycoprotein substrates in cynomolgus monkeys. J Pharmacol Exp Ther. 2014;350(3):578–88. https://doi.org/10.1124/jpet.114.214536.
Kathawala RJ, Gupta P, Ashby CR, Chen Z-S. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother 2015;18:1–17.
Durmus S, Hendrikx JJMA, Schinkel AH, Apical ABC. Transporters and cancer chemotherapeutic drug disposition. Adv Cancer Res. 2015;125:1–41. https://doi.org/10.1016/bs.acr.2014.10.001.
Kort A, Durmus S, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Brain and testis accumulation of regorafenib is restricted by breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm Res. 2015;32(7):2205–16. https://doi.org/10.1007/s11095-014-1609-7.
Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. https://doi.org/10.1017/S1462399411001888.
Malingré MM, Beijnen JH, Rosing H, Koopman FJ, Jewell RC, Paul EM, et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer. 2001;84(1):42–7. https://doi.org/10.1054/bjoc.2000.1543.
Sawicki E, Verheijen RB, Huitema ADR, van Tellingen O, Schellens JHM, Nuijen B, et al. Clinical pharmacokinetics of an amorphous solid dispersion tablet of elacridar. Drug Deliv. Transl. Res. 2017;7;125–131.
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. https://doi.org/10.1016/j.drup.2015.02.002.
Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013;102(9):3343–55. https://doi.org/10.1002/jps.23575.
Sane R, Mittapalli RK, Elmquist WF. Development and evaluation of a novel microemulsion formulation of elacridar to improve its bioavailability. J Pharm Sci. 2013;102(4):1343–54. https://doi.org/10.1002/jps.23450.
Sane R, Agarwal S, Elmquist WF. Brain distribution and bioavailability of elacridar after different routes of administration in the mouse. Drug Metab Dispos. 2012;40(8):1612–9. https://doi.org/10.1124/dmd.112.045930.
Tournier N, Goutal S, Auvity S, Traxl A, Mairinger S, Wanek T, et al. Strategies to inhibit ABCB1- and ABCG2-mediated efflux transport of erlotinib at the blood-brain barrier: a PET study in non-human primates. J Nucl Med. 2017;58(1):117–22. https://doi.org/10.2967/jnumed.116.178665.
Tong W-Q, Lee Wells M. Parenteral pharmaceutical compositions containing gf120918a. WO 1996011007 A1. http://www.google.com/patents/WO1996011007A1?cl=en. 1996 avr;
EMA. ICH guideline Q3C (R5) on impurities: guideline for residual solvents. 2015;
Katahira T, Teramoto K, Horiguchi S. [Experimental studies on the acute toxicity of tetrahydrofuran in animals]. Sangyō Igaku Jpn. J. Ind. Health. 1982;24:373–378.
Saubaméa B, Cochois-Guégan V, Cisternino S, Scherrmann J-M. Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab 2012;32:81–92, 1, DOI: https://doi.org/10.1038/jcbfm.2011.109.
Goutal S, Auvity S, Legrand T, Hauquier F, Cisternino S, Chapy H, et al. Validation of a simple HPLC-UV method for rifampicin determination in plasma: application to the study of rifampicin arteriovenous concentration gradient. J Pharm Biomed Anal. 2016;123:173–8. https://doi.org/10.1016/j.jpba.2016.02.013.
Karibe T, Hagihara-Nakagomi R, Abe K, Imaoka T, Mikkaichi T, Yasuda S, et al. Evaluation of the usefulness of breast cancer resistance protein (BCRP) knockout mice and BCRP inhibitor-treated monkeys to estimate the clinical impact of BCRP modulation on the pharmacokinetics of BCRP substrates. Pharm Res. 2015;32(5):1634–47. https://doi.org/10.1007/s11095-014-1563-4.
Acknowledgements
We thank Jérôme Cayla for helpful technical assistance during nonhuman primate experiments. Animal experiments were performed on a platform of France Life Imaging network partly funded by the grant “ANR-11-INBS-0006”. The Unité de Technologies Chimiques et Biologiques pour la Santé (UTCBS, CNRS UMR 8258, Inserm U1022. CNRS UMR 8258, Inserm U1022) is acknowledged for kind help regarding the characterization of the preparation.
Author information
Authors and Affiliations
Contributions
Characterization of the preparation was supervised by Karine Andrieux. She participated in the preparation, revision, and approval of the final manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Goutal, S., Langer, O., Auvity, S. et al. Intravenous infusion for the controlled exposure to the dual ABCB1 and ABCG2 inhibitor elacridar in nonhuman primates. Drug Deliv. and Transl. Res. 8, 536–542 (2018). https://doi.org/10.1007/s13346-017-0472-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0472-6