Abstract
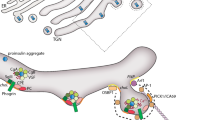
The sequence of events for secreting insulin in response to glucose in pancreatic β-cells is termed “stimulus-secretion coupling”. The core of stimulus-secretion coupling is a process which generates electrical activity in response to glucose uptake and causes Ca2+ oscillation for triggering exocytosis of insulin-containing secretory granules. Prior to exocytosis, the secretory granules are mobilized and docked to the plasma membrane and primed for fusion with the plasma membrane. Together with the final fusion with the plasma membrane, these steps are named the exocytosis process of insulin secretion. The steps involved in the exocytosis process are crucial for insulin release from β-cells and considered indispensable for glucose homeostasis. We recently confirmed a signature of defective exocytosis process in human islets and β-cells of obese donors with type 2 diabetes (T2D). Furthermore, cyclic AMP (cAMP) potentiates glucose-stimulated insulin secretion through mechanisms including accelerating the exocytosis process. In this mini-review, we aimed to organize essential knowledge of the secretory granule exocytosis and its amplification by cAMP. Then, we suggest the fatty acid translocase CD36 as a predisposition in β-cells for causing defective exocytosis, which is considered a pathogenesis of T2D in relation to obesity. Finally, we propose potential therapeutics of the defective exocytosis based on a CD36-neutralizing antibody and on Apolipoprotein A-I (ApoA-I), for improving β-cell function in T2D.



Similar content being viewed by others
References
Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–9. https://doi.org/10.1016/S2213-8587(18)30051-2.
Wagner R, Heni M, Tabak AG, Machann J, Schick F, Randrianarisoa E, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021;27(1):49–57. https://doi.org/10.1038/s41591-020-1116-9.
International Diabetes Federation. IDF Diabetes Atlas 2021. 10 ed. 2021.
Lawlor N, Khetan S, Ucar D, Stitzel ML. Genomics of Islet (Dys)function and Type 2 Diabetes. Trends Genet. 2017;33(4):244–55. https://doi.org/10.1016/j.tig.2017.01.010.
Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61(7):1726–33. https://doi.org/10.2337/db11-1516.
Gandasi NR, Yin P, Omar-Hmeadi M, Ottosson Laakso E, Vikman P, Barg S. Glucose-dependent granule docking limits insulin secretion and is decreased in human type 2 diabetes. Cell Metab. 2018;27(2):470–8. https://doi.org/10.1016/j.cmet.2017.12.017.
Nagao M, Esguerra JLS, Asai A, Ofori JK, Edlund A, Wendt A, et al. Potential protection against type 2 diabetes in obesity through lower CD36 expression and improved exocytosis in beta-cells. Diabetes. 2020;69(6):1193–205. https://doi.org/10.2337/db19-0944.
Andersson SA, Olsson AH, Esguerra JL, Heimann E, Ladenvall C, Edlund A, et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol Cell Endocrinol. 2012;364(1–2):36–45. https://doi.org/10.1016/j.mce.2012.08.009.
Seino S, Bell GI. Pancreatic Beta Cell in Health and Disease. Kaneohe: Springer; 2010.
Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087–97. https://doi.org/10.1369/jhc.5C6684.2005.
Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–45. https://doi.org/10.4161/isl.2.3.11815.
Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53.
Orci L, Gabbay KH, Malaisse WJ. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972;175(4026):1128–30. https://doi.org/10.1126/science.175.4026.1128.
Ivarsson R, Obermuller S, Rutter GA, Galvanovskis J, Renstrom E. Temperature-sensitive random insulin granule diffusion is a prerequisite for recruiting granules for release. Traffic. 2004;5(10):750–62. https://doi.org/10.1111/j.1600-0854.2004.00216.x.
Zhu X, Orci L, Carroll R, Norrbom C, Ravazzola M, Steiner DF. Severe block in processing of proinsulin to insulin accompanied by elevation of des-64,65 proinsulin intermediates in islets of mice lacking prohormone convertase 1/3. Proc Natl Acad Sci U S A. 2002;99(16):10299–304. https://doi.org/10.1073/pnas.162352799.
Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333(6168):93–6. https://doi.org/10.1038/333093a0.
Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, et al. Priming of insulin granules for exocytosis by granular Cl(-) uptake and acidification. J Cell Sci. 2001;114(Pt 11):2145–54.
Schnell AH, Swenne I, Borg LA. Lysosomes and pancreatic islet function A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell Tissue Res. 1988;252(1):9–15. https://doi.org/10.1007/BF00213820.
Dean PM. Ultrastructural morphometry of the pancreatic -cell. Diabetologia. 1973;9(2):115–9. https://doi.org/10.1007/BF01230690.
Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444(1–2):43–51. https://doi.org/10.1007/s00424-002-0781-5.
Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46(8):1029–45. https://doi.org/10.1007/s00125-003-1153-1.
Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol. 2008;586(14):3313–24. https://doi.org/10.1113/jphysiol.2008.155317.
Hutton JC, Penn EJ, Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210(2):297–305. https://doi.org/10.1042/bj2100297.
Rorsman P, Ashcroft FM. Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98(1):117–214. https://doi.org/10.1152/physrev.00008.2017.
Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57(6):1618–28. https://doi.org/10.2337/db07-0991.
Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann N Y Acad Sci. 2009;1152:187–93. https://doi.org/10.1111/j.1749-6632.2008.03992.x.
Eliasson L, Renstrom E, Ding WG, Proks P, Rorsman P. Rapid ATP-dependent priming of secretory granules precedes Ca(2+)-induced exocytosis in mouse pancreatic B-cells. J Physiol. 1997;503(Pt 2):399–412. https://doi.org/10.1111/j.1469-7793.1997.399bh.x.
Renstrom E, Eliasson L, Bokvist K, Rorsman P. Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol. 1996;494(Pt 1):41–52. https://doi.org/10.1113/jphysiol.1996.sp021474.
Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502(Pt 1):105–18. https://doi.org/10.1111/j.1469-7793.1997.105bl.x.
Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol. 2004;287(3):C565–71. https://doi.org/10.1152/ajpcell.00079.2004.
Straub SG, Shanmugam G, Sharp GW. Stimulation of insulin release by glucose is associated with an increase in the number of docked granules in the beta-cells of rat pancreatic islets. Diabetes. 2004;53(12):3179–83. https://doi.org/10.2337/diabetes.53.12.3179.
Barg S, Ma X, Eliasson L, Galvanovskis J, Gopel SO, Obermuller S, et al. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001;81(6):3308–23. https://doi.org/10.1016/S0006-3495(01)75964-4.
Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund PE, et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J Clin Invest. 2017;127(6):2353–64. https://doi.org/10.1172/JCI88491.
Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, et al. Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab. 2009;10(6):455–65. https://doi.org/10.1016/j.cmet.2009.09.011.
Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia. 2003;46(Suppl 1):M2-8. https://doi.org/10.1007/s00125-002-0930-6.
Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999;259(1–2):3–17. https://doi.org/10.1046/j.1432-1327.1999.00043.x.
Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30(11):564–72. https://doi.org/10.1016/j.tins.2007.08.008.
Tomas A, Meda P, Regazzi R, Pessin JE, Halban PA. Munc 18–1 and granuphilin collaborate during insulin granule exocytosis. Traffic. 2008;9(5):813–32. https://doi.org/10.1111/j.1600-0854.2008.00709.x.
Yin P, Gandasi NR, Arora S, Omar-Hmeadi M, Saras J, Barg S. Syntaxin clusters at secretory granules in a munc18-bound conformation. Mol Biol Cell. 2018;29(22):2700–8. https://doi.org/10.1091/mbc.E17-09-0541.
Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+ -dependent insulin exocytosis. J Cell Sci. 2004;117(Pt 15):3119–27. https://doi.org/10.1242/jcs.01179.
Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, et al. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105(10):3992–7. https://doi.org/10.1073/pnas.0711700105.
Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, et al. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115(2):388–96. https://doi.org/10.1172/JCI22955.
Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, et al. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem. 2003;278(11):9715–21. https://doi.org/10.1074/jbc.M211352200.
Tengholm A, Gylfe E. cAMP signalling in insulin and glucagon secretion. Diabetes Obes Metab. 2017;19(Suppl 1):42–53. https://doi.org/10.1111/dom.12993.
Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia. 2000;43(4):393–410. https://doi.org/10.1007/s001250051322.
Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155–79. https://doi.org/10.1146/annurev-physiol-030212-183754.
Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide 1 (7–36) amide stimulates exocytosis in human pancreatic beta-cells by both proximal and distal regulatory steps in stimulus-secretion coupling. Diabetes. 1998;47(1):57–65. https://doi.org/10.2337/diab.47.1.57.
Ammala C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363(6427):356–8. https://doi.org/10.1038/363356a0.
Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+. J Gen Physiol. 2004;124(6):653–62. https://doi.org/10.1085/jgp.200409082.
Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121(3):181–97. https://doi.org/10.1085/jgp.20028707.
Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004;53(Suppl 3):S59-62. https://doi.org/10.2337/diabetes.53.suppl_3.s59.
Seino S, Takahashi H, Takahashi T, Shibasaki T. Treating diabetes today: a matter of selectivity of sulphonylureas. Diabetes Obes Metab. 2012;14(Suppl 1):9–13. https://doi.org/10.1111/j.1463-1326.2011.01507.x.
Edlund A, Esguerra JL, Wendt A, Flodstrom-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12:87. https://doi.org/10.1186/1741-7015-12-87.
Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, et al. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297(2):E452–61. https://doi.org/10.1152/ajpendo.90585.2008.
Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFIIRim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277(52):50497–502. https://doi.org/10.1074/jbc.M210146200.
Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women The Nurses’ Health Study. Am J Epidemiol. 1997;145(7):614–9. https://doi.org/10.1093/oxfordjournals.aje.a009158.
Olofsson CS, Salehi A, Holm C, Rorsman P. Palmitate increases L-type Ca2+ currents and the size of the readily releasable granule pool in mouse pancreatic beta-cells. J Physiol. 2004;557(Pt 3):935–48. https://doi.org/10.1113/jphysiol.2004.066258.
Nagao M, Asai A, Inaba W, Kawahara M, Shuto Y, Kobayashi S, et al. Characterization of pancreatic islets in two selectively bred mouse lines with different susceptibilities to high-fat diet-induced glucose intolerance. PLoS ONE. 2014;9(1): e84725. https://doi.org/10.1371/journal.pone.0084725.
Nagao M, Esguerra JLS, Wendt A, Asai A, Sugihara H, Oikawa S, et al. Selectively Bred Diabetes Models: GK Rats, NSY Mice, and ON Mice. Methods Mol Biol. 2020;2128:25–54. https://doi.org/10.1007/978-1-0716-0385-7_3.
Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48(9):1686–90. https://doi.org/10.2337/diabetes.48.9.1686.
Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21(9):2255–69. https://doi.org/10.1210/me.2007-0077.
Nilsson O, Del Giudice R, Nagao M, Gronberg C, Eliasson L, Lagerstedt JO. Apolipoprotein A-I primes beta cells to increase glucose stimulated insulin secretion. Biochim Biophys Acta Mol Basis Dis. 2020;1866(3): 165613. https://doi.org/10.1016/j.bbadis.2019.165613.
Saito H, Dhanasekaran P, Nguyen D, Deridder E, Holvoet P, Lund-Katz S, et al. Alpha-helix formation is required for high affinity binding of human apolipoprotein A-I to lipids. J Biol Chem. 2004;279(20):20974–81. https://doi.org/10.1074/jbc.M402043200.
Edmunds SJ, Liebana-Garcia R, Nilsson O, Domingo-Espin J, Gronberg C, Stenkula KG, et al. ApoAI-derived peptide increases glucose tolerance and prevents formation of atherosclerosis in mice. Diabetologia. 2019;62(7):1257–67. https://doi.org/10.1007/s00125-019-4877-2.
Edmunds SJ, Liebana-Garcia R, Stenkula KG, Lagerstedt JO. A short peptide of the C-terminal class Y helices of apolipoprotein A-I has preserved functions in cholesterol efflux and in vivo metabolic control. Sci Rep. 2020;10(1):18070. https://doi.org/10.1038/s41598-020-75232-0.
Eliasson L, Esguerra JLS, Wendt A. Lessons from basic pancreatic beta cell research in type-2 diabetes and vascular complications. Diabetol Int. 2017;8(2):139–52. https://doi.org/10.1007/s13340-017-0304-4.
Asplund O, Storm P, Chandra V, Ottosson-Laakso E, Hatem G, Mansour-Aly D, et al. Islet Gene View - a tool to facilitate islet research. BioRxiv. 2020. https://doi.org/10.1101/435743.
Alonso L, Piron A, Moran I, Guindo-Martinez M, Bonas-Guarch S, Atla G, et al. TIGER: The gene expression regulatory variation landscape of human pancreatic islets. Cell Rep. 2021;37(2): 109807. https://doi.org/10.1016/j.celrep.2021.109807.
Acknowledgements
We thank our present and former colleagues at Lund University Diabetes Centre (LUDC) and Nippon Medical School for contributions to the research introduced in this review and Yoshimi Nagao for supporting figure illustration. We acknowledge the human tissue laboratory at LUDC/Exodiab and the Nordic Centre for Islet Transplantation for the delivery of islets from human donors. We are grateful for support to our research in this area from the JSPS KAKENHI Grant Numbers 17KK0184, 19K23872 and 21K05453, the European Foundation for the Study of Diabetes and the Japan Diabetes Society, the Diabetes Wellness Sverige, the Uehara Memorial Foundation, the Scandinavia-Japan Sasakawa Foundation, the Sumitomo Life Welfare Foundation, the Ono Medical Research Foundation, the Swedish Research Council (LE project grant, JOL project grant, and an SRA grant SFO-EXODIAB (2009-1039)), ALF-Region Scania, Sweden, The Swedish Diabetes Foundation and the Swedish Foundation for Strategic Research (IRC-LUDC; IRC15-0067).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
JOL is an employee of Novo Nordisk A/S, DK. The authors have no conflict of interest.
Ethical statement
The work using human islets was approved by the ethics committee in Malmö/Lund, Sweden: project No. 2011/263, date of approval 2011–05-03. All the procedures on the human islets were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Donors or their relatives had given their written consent to donate organs for biomedical research upon admission to the intensive care unit.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nagao, M., Lagerstedt, J.O. & Eliasson, L. Secretory granule exocytosis and its amplification by cAMP in pancreatic β-cells. Diabetol Int 13, 471–479 (2022). https://doi.org/10.1007/s13340-022-00580-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-022-00580-3