Abstract
Background and Objectives
Probability of target attainment (PTA) curves are commonly used to support dose recommendations of antibiotics for different patient groups. In this study we propose PTA analysis to optimize sugammadex dosing in children.
Methods
This study involved data from an observational cohort study of 30 American Society of Anesthesiologists (ASA) Physical Status I and II children undergoing surgery requiring muscle relaxation. All patients received 0.6 mg/kg rocuronium, with sugammadex administered at the end of surgery in three different doses (0.5, 1.0, and 2.0 mg/kg) to reverse the neuromuscular blockade.
Results
The data were analyzed using a population Bayesian-based approach. The developed model was used to simulate pharmacokinetic–pharmacodynamic profiles for different patient groups and dosing regimens before the PTA analysis was performed to translate these simulations into a clinically useful measure. The target was defined as neuromuscular blockade reversal measured by Train-of-Four (TOF ratio > 90%) at 1.5, 3, and 5 min post sugammadex dose. The sugammadex doses leading to 90% PTA were determined for different patients’ body weights, rocuronium doses, and time gaps between rocuronium and sugammadex administration assuming the model, priors, and gathered data. For comparison, PTA curves for a range of clinical scenarios are provided to illustrate the usefulness of PTA analysis in selecting the appropriate dose for a given patient.
Conclusions
The proposed PTA analysis is useful to support the sugammadex dose selection in different clinical scenarios.
Trial Registration
The study was registered by ClinicalTrials.gov under number NCT04851574 on 21 April 2021.
Similar content being viewed by others
This study describes the development and evaluation of a Bayesian-based population pharmacokinetic–pharmacodynamic model of rocuronium and sugammadex in children undergoing surgery. |
This study demonstrates the usefulness of the probability of target attainment analysis in supporting sugammadex dose selection based on the body mass of the child, rocuronium dose, and the time of sugammadex administration after the last rocuronium dose. |
1 Introduction
Sugammadex (SGX) is a modified gamma-cyclodextrin designed to encapsulate aminosteroid muscle relaxants, such as rocuronium (ROC) and vecuronium. Sugammadex administration results in rapid reversal of the neuromuscular blockade (NMB) via a noncompetitive mechanism in a dose-dependent manner [1, 2]. During the registration process, as well as following sugammadex approval, many clinical trials have investigated its safety, effectiveness, and optimal dosing strategies [3,4,5,6,7,8] in adults as well as in a pediatric population. A retrospective study covering the full age range of children demonstrated the safety and effectiveness of sugammadex reversal of NMB in children [9]. Compared with neostigmine, the sugammadex reversal of NMB was associated with less bradycardia in older children and adolescents, with a shorter end-interval time in neonates [9]. In a randomized controlled trial, sugammadex administration in children resulted in rapid recovery after general anesthesia, faster extubation times, and less incidence of adverse events compared with neostigmine [10].
The USA and European Union (EU) drug agencies have made different regulatory decisions regarding sugammadex [11]. Sugammadex is approved in the USA for the reversal of NMB induced by rocuronium or vecuronium in adults (not children) undergoing surgery, whereas the drug is also approved for use in pediatric patients aged 2–17 years in the EU [11]. The under-dosing strategy of sugammadex has recently been suggested as a potential cost-saving strategy in the reversal of deep NMB, but the usefulness of this strategy is still under debate [12]. Unquestionably, the population pharmacokinetic–pharmacodynamic (PK/PD) models and simulations for various patient groups might be useful to assess the impact of this under-dosing strategy. Population pharmacokinetic–pharmacodynamic approaches are especially helpful for sparse and unbalanced datasets that are common in studies involving pediatric populations [13].
Several pharmacokinetic and pharmacodynamic models of rocuronium/vecuronium and sugammadex have been published [14,15,16,17]; however, the data and models are still limited for the pediatric population. Ploeger et al. [14] developed a mechanistic pharmacokinetic–pharmacodynamic model for the sugammadex reversal of rocuronium-induced NMB, which implemented an equilibrium dissociation constant (Kd) for the rocuronium–sugammadex complex. This concept was then extended by Kleijn et al. [16], who developed the more comprehensive population pharmacokinetic–pharmacodynamic model of the rocuronium–sugammadex interaction. Such population pharmacokinetic–pharmacodynamic models allow the construction of probability of target attainment (PTA) curves. PTA analysis is extensively used to identify and optimize dosing regimens during antibiotic development and to guide specific dosing recommendations in different patient populations [18]. Similarly, it can be a tool to optimize the dosing regimens of sugammadex by calculating the proportion of subjects achieving the desired target (e.g., desired reversal time).
This study aimed to predict the minimum effective dose of sugammadex in a population of healthy American Society of Anesthesiologists (ASA) Physical Status I and II children, based on PTA analysis. For this purpose, a population Bayesian-based pharmacokinetic–pharmacodynamic model was developed for rocuronium-induced NMB and its reversal by sugammadex given in three dosing schemes of 0.5, 1, and 2 mg/kg, two of which were subtherapeutic (0.5 and 1 mg/kg). The final population model was used to simulate PK/PD time profiles for selected dosing regimens and selected patient groups (e.g., with different body weights) to determine the PTA and the minimum effective dose.
2 Methods
2.1 Patients
This study was a prospective cohort study and the research protocol was approved by the local Ethics Committee. The study was registered in ClinicalTrials.gov—study number NCT04851574. Written informed consent was obtained from parents and in the case of children over 16 years of age, also from the patients. The patients were enrolled to the study between January 2018 and December 2019. The study sample was limited to children from 2 to 18 years of age of both sexes, having ASA Physical Status I or II, with general anesthesia during the operation requiring muscle relaxation for more than 20 min.
All children received oral premedication of 0.2 mg/kg midazolam and the introduction to general anesthesia depended on the presence of an intravenous cannula, with patients receiving fentanyl (1 µg/kg) and propofol (2–4 mg/kg) according to the patient’s age. Patients without an intravenous cannula underwent volatile induction with sevoflurane at a dose of 5–6 vol%. After catheterization, the anesthesia for all patients was maintained by continuous infusion of propofol to exclude any influence of sevoflurane under our implemented protocol [19], according to which children aged 2–5 years received 4 mg/kg of propofol for induction, followed by infusion at 17 mg/kg/h for the first 30 min, 12 mg/kg/h for the next 30 min, and 10 mg/kg/h until the end of the operation. Similarly, children aged 6–11 years received 3 mg/kg of propofol for induction, followed by infusion at 15 mg/kg/h for the first 30 min, 10 mg/kg/h for the next 30 min, and 8 mg/kg/h until the end of the operation. Adolescents aged ≥ 12 years received 2 mg/kg of propofol for induction, followed by the infusion at 10 mg/kg/h for the first 30 min, 8 mg/kg/h for the next 30 min, and 6 mg/kg/h until the end of the operation. For all patients, rocuronium was administered at a dose of 0.6 mg/kg to facilitate anesthesiologist endotracheal intubation and the maintenance of muscle relaxation for surgical procedures. During the operation, anesthesia was supplemented by additional doses of fentanyl and the next dose of rocuronium (half of the first initial dose) administered when the TOF ratio exceeded 40%. During anesthesia, patients were monitored continuously by ECG, noninvasive blood pressure, pulse oximetry, capnometry, temperature, and TOF ratio.
After the end of the operation, the neuromuscular blockade was reversed by sugammadex administered in doses of 0.5, 1.0, and 2 mg/kg. One patient who received the minimal dose of 0.5 mg/kg of sugammadex was excluded from the study because of a paradoxical reaction to the drug. After this dose, TOF ratio increased only in 10–15%, so the decision was to give the same second dose of sugammadex, and to our amazement the TOF ratio dropped to 0%. This unexpected response to the drug will be presented as a case study. For this reason, the study was continued and limited to two doses of sugammadex of 1.0 and 2 mg/kg.
Neuromuscular monitoring is recommended after administration of muscle relaxant. In this work, the TOF ratio was measured by an Infinity Trident NMT SmartPod (Drägerwerk AG & Co. KGaA, Moislinger Allee 53-55,23558 Lübeck, Germany). The method used in our study was T4/T1. The sensor was placed on the thumb of the patient. Two stimulating electrodes were placed along the ulnar nerve together with the special temperature electrode. After the completion of the equipment set-up, calibration was performed according to the company’s instruction. TOF ratio data were collected every 20 s during the first 2 min and every 1 min from 2 to 5 min after rocuronium administration. During the operation, values were recorded at 5- or 10-min intervals and just before sugammadex administration. After sugammadex administration, the TOF ratio was measured at the same time points as after rocuronium administration.
The reading of the TOF ratio value allowed for the ongoing assessment of the degree of neuromuscular blockade to determine the potential need for a maintenance dose of myorelaxant, as well as to recognize the moment at which optimal conditions for endotracheal intubation were reached. In turn, after administration of sugammadex, the TOF ratio allowed the patient to be extubated under safe conditions. In line with the current anesthesia recommendations, a TOF ratio of 90% was considered as the reversal time, providing safe conditions for extubation [20]. With this parameter, episodes of recurarization were also recorded, i.e., when three consecutive TOF ratio readings showed a decrease from 90 to less than 80. The mean arterial pressure (MAP), systolic (SBP), and diastolic (DBP) blood pressure, and heart rate (ECG) were recorded as standard monitoring and as a warning of possible episodes of intraoperative bradycardia, tachycardia, hypotension, and/or hypertension.
During the surgery, blood samples (0.7 ml) were collected from the vein at three time points: 2 and 15 min after administration of rocuronium, and immediately before sugammadex dose. Patient blood samples (2 ml) were also collected 2, 5, 15, 60 min, and 6 h after sugammadex administration. The blood was placed in heparinized tubes and transferred to the laboratory for centrifugation and storage at –20 °C.
2.2 Analytical Methods
Plasma rocuronium and sugammadex concentrations were determined using validated chromatographic methods with mass spectrometric detection (LC-MS/MS), in full compliance with good laboratory practice regulations. An Exion LC system (Applied Biosystems/Sciex, Massachusetts, USA) consisted of two chromatography pumps, a degasser, an autosampler (a CTC Pal liquid sampler) and a temperature-controlled compartment for the column (column oven). Detection of analytes and internal standards was operated on triple-quadrupole mass spectrometer, Sciex 4500 QTRAP (Applied Biosystems/Sciex, Massachusetts, USA), equipped with a TurboIonSpray interface and the Analyst software version 1.6.3. Chromatographic separations were performed by Jupiter 5 µm C4 300 Å, 50 × 2 mm, on an Ea (Phenomenex, Torrance, CA, USA) LC column. The following chromatographic conditions were applied: gradient method with mobile phase A: 0.1% HCOOH in H20, mobile phase B: 0.1% HCOOH in acetonitrile, the initial mobile phase composition was maintained at 5% solvent A for 1 min, changed linearly to 30% (1–3 min), then to 90% (3–4 min), then held 1 min at 90%, then returned to the initial conditions and was kept at 2 min for the chromatographic column to equilibrate. The oven temperature was 50 °C, flow rate was 0.8 ml/min, and the injection volume was 5 µl. Rocuronium and sugammadex were analyzed using the same gradient and on the same run.
During sample preparation, 100 µl of serum was mixed with 10 µl of deuterated sugammadex (100 µg/ml) and precipitated in two steps. First, with 10 µl of trichloroacetic acid and mixed by vortexing for 10 s (3500 rpm) and 5 min (1200 rpm). Second, by adding 100 µl of methanol and mixed on the vortex for 10 s (3500 rpm) and 5 min (1200 rpm). The precipitated sample was centrifuged for 5 min at 13,000 rpm and diluted 20 times with HPLC grade water. All samples were analyzed in one batch within 1 day.
Analyses were conducted in the positive ionization mode for rocuronium and negative for sugammadex. The optimized source parameters were as follows: 20 psi for curtain gas; 8 psi for collision activation dissociation; 600 °C for turbo heater temperature; 40 psi for gas 1; 35 psi for gas 2. The multiple reaction monitoring mode was used to determine three transitions for rocuronium, sugammadex, and the internal standard (deuterated sugammadex). Calibration curves and quality control samples were prepared on blank plasma. The eight-point calibration curves for rocuronium were obtained in the range of 40–1000 ng/ml with a correlation coefficient r > 0.9994 and in the range of 4–10,000 ng/ml with a correlation coefficient of 0.9965 for results under 40 ng/ml. Quality control samples for rocuronium were at the level of 100 and 200 ng/ml. Recovery was 69.9% and 99.9% and relative standard deviation was 4.6% and 13%, respectively. The calibration curves, quality control samples, and samples for rocuronium quantification were prepared using peak areas without internal standard owing to the lack of isotopic internal standard. The calibration curve for sugammadex was obtained in the range of 0.1–100 µg/ml with a correlation coefficient r > 0.9995 using analyte/internal standard (deuterated sugammadex) ratio. Quality control samples for sugammadex were at the level of 2.5 and 5 µg/ml. Recovery was 75.5% and 86.3% and relative standard deviation was 3.3% and 1.7%, respectively. The analytical procedure was validated according to Food and Drug Administration (FDA) guidelines [21], and all validation steps confirmed that the applied analytical procedure was appropriate for the measurement of rocuronium and sugammadex plasma concentrations.
2.3 Pharmacokinetic–Pharmacodynamic Model
Population modeling was performed using NONMEM (version 7.4.4, ICON Development Solutions, Ellicott City, MD, USA) and the Fortran compiler 9.0. NONMEM runs were executed using Wings for NONMEM (WFN740, http://wfn.sourceforge.net). A fully conditional hierarchical Bayesian analysis was conducted using NONMEM with the $PRIOR NWPRI statement [22]. NONMEM data processing, simulations, and plots were conducted using Matlab Software version 7.0 (MathWorks Inc., Natick, MA, USA). Four MCMC chains were run for 2000 samples (the first 1000 burn in samples per chain were discarded). Thus 4000 samples were used to make an inference about the posterior distributions of model parameters. The trace plots were used to assess whether the chains had reached stationary distribution. The data were analyzed using the following strategy:
-
1.
The structural model developed by Kleijn et al. [16] was selected to describe the available data.
-
2.
Pharmacokinetic parameters were adjusted from adults to children based on the principles of allometry/isometry scaling. Since children in this study had normal renal clearance, the body weight was assumed to be the only factor explaining the size-related effects in this patient group.
-
3.
The pharmacokinetic parameters were standardized to a 70 kg subject, allowing the utilization of prior knowledge about model parameters given by Kleijn et al. [16].
The structural model described the free rocuronium (CROC), free sugammadex (CSGX), and the rocuronium–sugammadex complex using two-compartmental models (parametrized using VR1, VR2, CLR1, CLR2 for rocuronium volumes of distributions and clearances, and VS1, VS2, CLS1, CLS2 for sugammadex volumes of distributions and clearances). Complexation was assumed to occur in the central compartments. Binding was described using the rate constants for association (kon) and dissociation (koff), with the dissociation constant (Kd) included as a fixed parameter (0.0559 mM). A biophase compartment (with rate keo) was used to introduce the delay between rocuronium concentrations and TOF ratio measurements. The TOF ratio measurements were linked to the biophase rocuronium concentrations via an Emax model that was parametrized using baseline TOF ratio (E0 : fixed to 100), a maximal reduction in TOF ratio (Emax: fixed to 100), the rocuronium concentration in the biophase compartment leading to a 50% reduction in TOF ratio (EC50,R), and the Hill coefficient (GAM). An additional route of elimination of rocuronium from the effect compartment was modeled by a second-order process with a rate constant (ks) and was proportional to the rocuronium biophase concentrations and the free sugammadex concentration in the central sugammadex compartment. The concentration were converted to molar concentrations using molecular weights of 527.8 g/mol for rocuronium and 2002.2 g/mol for sugammadex. The graphical representation of the model is presented in Fig. 1 in Kleijn et al. [16].
Interindividual variability (IIV) for all pharmacokinetic-pharmacodynamic parameters was modeled assuming log-normal distribution (normal distribution on a logarithmic scale) (Eqs. 1 and 2):
where \({P}_{\mathrm{PK},i}\) and \({P}_{\mathrm{PD},i}\) denote a set of pharmacokinetic and pharmacodynamic parameters for the ith subject (the parameters are characterized in Table S1, Supplementary material 2), \({\theta }_{P,\mathrm{PK}}\) and \({\theta }_{P,\mathrm{PD}}\) denote a set of typical values of population parameters, BWi denotes the individual body weight, where 70 kg is a typical adult body weight, and K is the exponent equal to 0.75 for clearance, 1 for volumes of distribution , –0.25 for rates (keo and ks), and 0 for other pharmacokinetic–pharmacodynamic parameters. \({\eta }_{P,\mathrm{PK},i}\) and \({\eta }_{P,\mathrm{PK},i}\) denote random effects for model parameters and were assumed to follow a multivariate normal distribution (MVN) mean 0 and variance–covariance matrix \({\Omega }_{\mathrm{PK}}\) and \({\Omega }_{\mathrm{PD}}\) (Eqs. 3 and 4):
A tilde (∼) denotes “has the probability distribution of” (i.e., the values of \({\eta }_{P,i}\) are randomly drawn from MNV distribution).
Any jth observation for the ith individual, CROC,ij, CSGX,ij and TOFij at time tj, was defined as follows (Eqs. 5–7):
where \({C}_{\mathrm{ROC}}, {C}_{\mathrm{SGX}}, \mathrm{TOF}\) are defined by the basic structural model and \({\varepsilon }_{\mathrm{ROC},ij}\) and \({\varepsilon }_{\mathrm{SUG},ij}\) represents the proportional random error for pharmacokinetic measurements, and \({\varepsilon }_{\mathrm{TOF},ij}\) represents an additive random error for pharmacodynamic measurements. It was assumed that \(\varepsilon\) is normally distributed with mean 0 and variance \({\sigma }^{2}\) (Eqs. 8–10):
The priors were assumed as follows (Eqs. 12 and 13):
The priors consisted of the vector of hyperprior population mean parameters,\({ \mathrm{log}\theta }_{P,0}\) and its variances \(\Sigma\), the between-subject variance-covariance matrix Ω was assumed to follow an inverse Wishart distribution with \({\Omega }_{0} \mathrm{and}\) degrees of freedom \({\mathrm{d}f}_{\Omega }\). The inverse Wishart distribution was also used for sigma. The numerical values for \({\mathrm{log}\theta }_{P,0}\),\(\Sigma , { \Omega }_{0}, {\mathrm{d}f}_{\Omega }{, \sigma }_{0}, \mathrm{d}{f}_{\sigma }\) are provided in Table S1 (Supplementary Material 2). Briefly, the hyperprior population mean pharmacokinetic–pharmacodynamic parameters were set to values provided by Kleijn et al. [16]. The standard deviations were assumed to be 5% for pharmacokinetic parameters and 30% for pharmacodynamic parameters. The informative prior were used to model between-subject variability that assumed an %CV equal to 30% with 20 degrees of freedom. The uninformative priors were assumed for residual errors with mean values equal to those obtained in [16] and low degrees of freedom. Please note that the prior uncertainty is slightly higher than reported in the literature and was used mainly to provide marginal information about model parameters. Consequently, it allowed stabilization of the estimation of model parameters. The data and model code is given in Supplementary Material 1 and 2 (S2).
2.4 Simulations
The model and obtained posterior distribution of model parameters (n = 4000) was used to simulate pharmacokinetic–pharmacodynamic profiles expected for a subject with a certain set of covariates (i.e., body weight) under a certain dosing regimen. As an illustration, the pharmacokinetic–pharmacodynamic profile for a 20 kg subject receiving 0.6 mg/kg rocuronium followed by 0.5, 1, and 2 mg/kg of sugammadex administered 20 min post rocuronium dose was simulated. The outcomes of these simulations were represented as 50th (median) and (5–95th) percentiles to illustrate the typical pharmacokinetic–pharmacodynamic profiles, along with the uncertainty to assess the clinical response by calculating the probability of achieving TOF ratio > 90% at any given time.
The PTA analysis was performed to translate the simulation into a clinically useful measure. The target was defined as a reversal time of 1.5, 3, and 5 min post sugammadex dose, with posterior pharmacokinetic–pharmacodynamic parameters for a given subject used to simulate a range of plausible TOF ratio profiles for the desired dosing regimen based on pharmacokinetic–pharmacodynamic equations. The reversal time (time post sugammadex administration after which TOF ratio > 90% was observed) was determined for each generated pharmacokinetic–pharmacodynamic profile. Finally, the probability representing the fraction of simulated subjects (profiles) achieving that target was plotted for a range of sugammadex doses and administration times.
3 Results
3.1 Patients
Data were collected from 30 patients of both sexes (22 males/8 females) with a median (range) age of 8.5 years (2.5–17 years) and body weight of 36.5 kg (14–85 kg). In this group, 19 and 11 patients were of ASA Physical status I and II, respectively.
3.2 Drug Concentrations and TOF Ratio Measurements
A total of 223 and 130 blood samples were collected to determine rocuronium and sugammadex plasma concentrations, respectively. The data consisted of 992 TOF ratio measurements, with individual concentration–time profiles and the resulting TOF ratio values provided in Fig. 1.
3.3 Pharmacokinetic Model and Covariate Testing
The data were analyzed assuming a literature pharmacokinetic–pharmacodynamic model of rocuronium and sugammadex. The informative priors were used to take external knowledge about model parameters into account. The summary of a marginal a posteriori distribution of model parameters is provided in Table S1 (Supplementary Material 2). The overall similarities of prior and posterior parameters suggest the similarity of pharmacokinetic–pharmacodynamic profiles between the present and previous studies [16].
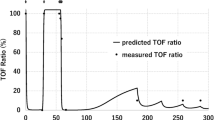
The experimental data and final model fittings for plasma concentrations and TOF ratios are shown in Figs. 2 and 3 for a representative subset of subjects. The predictions (along with uncertainties) are also shown in Supplementary Material 2 (S3) for all subjects. The model fittings show that the final pharmacokinetic–pharmacodynamic model accurately described measurements. The goodness-of-fit diagnostic plots for the final model are presented in Supplementary Material 2 (S4). The individual and population predictions versus observed concentrations and TOF ratio measurements are relatively symmetrically distributed around the line of identity, although there is some indication of bias in population predictions of TOF ratio, nevertheless the conditional weighted residuals versus population predicted concentrations and versus time do not show any trend and are approximately normally distributed around zero, indicating reasonable calibration of the model with the available data. For diagnostic purpose, we provide ETA plots in Supplementary Material 2 (S5) that present mean a posteriori estimates of ETA (deviation of the individual estimate from the population mean on a logarithmic scale) and body weight, age, and sex. Since we are using a fairly strong prior for typical values of pharmacokinetic–pharmacodynamic parameters, some miscalibration can be attributed to the fact that model predictions are shrunk toward the prior predictions.
3.4 Simulations
Figure 4 presents the typical pharmacokinetic–pharmacodynamic profile along with the uncertainty expected for a 20 kg subject receiving 0.6 mg/kg dose of rocuronium followed by 0.5, 1, and 2 mg/kg of sugammadex administered 20 min post rocuronium dose. The probability of achieving TOF ratio > 90% was plotted to visualize the clinical response (reversal of rocuronium action), with the TOF ratio value increasing in a dose-dependent manner after sugammadex administration. The 2 mg/kg sugammadex dose led to less than 2 min reversal time for most subjects, with lower sugammadex doses leading to more uncertain reversals. Similar figures for subjects with a range of body weights and times of sugammadex administrations post rocuronium dose are presented in Supplementary Material 2 (S6).
The simulated concentrations of rocuronium and sugammadex along with corresponding TOF ratio values for a 20 kg subject receiving a 0.6 mg/kg rocuronium dose followed by 0, 0.5, 1, and 2 mg/kg of sugammadex administered 20 min post rocuronium dose. The lines denote the mean and areas cover the 5–95th percentiles. The bottom panel presents the clinical response represented as % subjects (body weight of 20 kg) with TOF ratio higher than 90% at a given time. TOF Train-of-Four, BW body weight, SGX sugammadex
Based on these plots, PTA curves were constructed to better understand the impact of sugammadex dose and administration time on reversal time for subjects of different body weights (Fig. 5), showing the probability of reversal time being less than 3 min post sugammadex dose (where reversal time corresponds to the time the subject achieves TOF ratio > 90%). Based on simulations, the reversal time from sugammadex administration to a TOF ratio of 90% for patients with a range of body weights, times of sugammadex administration, and sugammadex doses is presented in Table 1. The sugammadex dose leading to the 90% probability of target attainment is given in Tables 2 and 3. As an example, 90% of 10 kg patients are expected to reach the target (reversal time of 3 min) after 1.1 mg/kg sugammadex given 20 min post rocuronium dose. For these patients, the sugammadex dose should be increased to 2.9 mg/kg if sugammadex is given 2 min after rocuronium. Similarly, 90% of 70 kg patients are expected to reach the same target for a 2.0 mg/kg dose given 20 min post rocuronium. For these patients, the sugammadex dose needs to be increased to 3.8 mg/kg if sugammadex is given 2 min after rocuronium.
The probability of reversal time being less than 3 min post sugammadex dose (reversal time corresponds to time with TOF ratio > 90%) for different sugammadex doses, sugammadex administration times, and patient body weights. Rocuronium was administered at a dose of 0.6 mg/kg and sugammadex was administered at a dose ranging from 0.5 to 4 mg/kg, 2, 5, 10, and 20 min after the rocuronium dose. BW body weight, SGX sugammadex
4 Discussion
This work proposed PTA analysis for sugammadex dosing optimization in ASA Physical Status I and II children. According to the product information [23], recommended sugammadex doses for routine reversal of neuromuscular blockade are 4 and 2 mg/kg for adults and children, respectively (with median TOF ratio recovery to 90% around 3 min). For rescue use of sugammadex and immediate reversal of rocuronium blockade, recommendations are available for adults only, suggesting sugammadex dose of 16 mg/kg (with median TOF ratio recovery to 90% of around 1.5 min). Owing to limited experience with the use of sugammadex in infants (30 days–2 years) and a lack of studies in term newborn infants (less than 30 days), the use of sugammadex in these patients is not recommended until further data become available. Similarly, an immediate reversal in children and adolescents has not been investigated, and for this reason, sugammadex is not recommended until now [24].
Since sugammadex was introduction to clinical practice, lower than recommended doses (e.g., 0.5–1.0 mg/kg) have been used and reported, mainly as a cost-saving strategy. However, the under-dosing strategy may lead to an increased risk of recurrence of neuromuscular block after initial reversal [12]. Patient age has also been shown to significantly influence the reversal time after sugammadex administration [16], thus a deeper understanding of the dose-effect relationship taking into account the patient’s age/size and different clinical scenarios is needed to optimize sugammadex dosing. In this study, we focused on the pediatric population (2–17 years old) since pharmacokinetic–pharmacodynamic data of sugammadex and rocuronium in children is less available than in adults. It was hypothesized that lower doses might be sufficient in some clinical scenarios in children. Owing to the complexity of the pharmacokinetic–pharmacodynamic model of the rocuronium–sugammadex complex, as well as sparse clinical data, we used the Bayesian approach utilizing the published population pharmacokinetic–pharmacodynamic model [16]. This model was developed based on data from several clinical studies in adults and one in infants and children. Full Bayesian analysis utilizing the knowledge available in the literature was performed to better calibrate our data with the model predictions. The analysis consisted of several steps: (1) selection of the pharmacokinetic–pharmacodynamic model, (2) elucidation of prior information on model parameters from literature, (3) model inference, (4) an exploratory assessment of covariate relationship explaining interindividual and intraindividual differences in drug pharmacokinetics and pharmacodynamics, (5) identification of differences between literature-described patients and those enrolled in this study, and (6) simulation-based assessment of the impact of sugammadex dose on reversal time.
4.1 Clinical simulations
The developed model was used to predict reversal time for 10, 20, 40, and 70 kg subjects administered with a range of sugammadex doses 2, 5, 10, and 20 min post rocuronium dose of 0.6 mg/kg. The provided simulation corresponds to the most common clinical condition. Based on these simulations, it can be concluded that sugammadex administration at a dose of 4 mg/kg leads to a reversal of rocuronium blockade in less than 3 min, irrespective of subject weight and time of sugammadex administration. One can also expect a decrease in the reversal time for patients with lower body weight, delayed sugammadex administration, and increased sugammadex dose.
During elective anesthesia, rocuronium is usually used at a dose of 0.6 mg/kg and the reversal of NMB after cessation of surgical procedure should be as fast as possible. For infants with a body weight of 5 kg, the sugammadex dose of 4 mg/kg leads to TOF ratio > 90% within 3 min, even if rocuronium is administered 2 or 5 min before sugammadex doses. Lower doses are less effective and the NMB reversal could be expected later, possibly 33 (90% CI 4–88), 21 (90% CI 1–71), or 7 (90% 0–35) min for sugammadex doses of 0.5, 1, and 2 mg/kg, respectively, administered 2 min after rocuronium dose.
During controlled standard anesthesia, sugammadex is often given 20 min after the last rocuronium dose, and a dose of 1.1 mg/kg should be sufficient for 5 kg infants (if aiming for a reversal time of 3 min). A 0.5 mg/kg sugammadex dose would lead to a prolonged reversal time (> 3 min) in a relatively high proportion of subjects (about 30%). The dose can be lowered but only if longer reversal times are possible (e.g., reversal time of 5 min). In children with higher body weight (10, 20, or 40 kg of body mass), the sugammadex dose of at least 4 mg/kg allows TOF ratio > 90% to be achieved in 2–3 min on average (less than 6 min for most subjects). For lower doses of 0.5, 1, or 2 mg/kg of sugammadex, much longer reversal times are likely (Table 1). In children approaching the adult weight of 70 kg, this time of reversal will be longer and the recommended sugammadex dose of 2 mg/kg would reverse blockade after 15 (0–75), 11 (0–63), 7 (0–36), and 4 (0–9) min on average (90% confidence) if given 2, 5, 10, and 20 min after rocuronium administration, respectively.
Immediate reversal of neuromuscular blockade within 3 min after administration of rocuronium is used in an emergency. In this case, the dose of 16 mg/kg sugammadex is recommended for adults, but there are no such recommendations available for children [25, 26]. According to the standard reversal, rescue sugammadex doses should be able to accelerate the speed of recovery from the neuromuscular block to a TOF ratio of 90% in an average of 3 min [27]; this will be shorter for younger children [28].
Based on simulations, for children with unanticipated difficult intubation following the standard 0.6 mg/kg rocuronium dose, the rescue sugammadex bolus at a dose of 3.3, 3.6, 4.1, 4.5, or 4.8 mg/kg should be sufficient for patients weighing 5, 10, 20, 40, or 70 kg, respectively. When the time of reversal of NMB needs to be reduced to 1.5 min in the youngest and/or the sickest children, then the required sugammadex dose should be almost doubled to 5.3, 6.0, 6.7, 7.4, and 7.9 mg/kg based on the same patient body mass.
In another scenario of predictable difficult intubation (e.g., congenital defects, traumatic injuries) when the rocuronium dose of 1.2 mg/kg is recommended, immediate reversal of blockade needs higher sugammadex doses in all age groups from 6.5 mg/kg for a 5 kg infant to 8.3 mg/kg for a 40 kg child, and even 9 mg/kg for patients weighing 70 kg [29]. Similarly, shortening the time to 1.5 min requires a further increase in the dose by a factor of 1.5, which means an effective dose for an infant is 8.8 mg/kg and for a teenager is 12–13 mg/kg of sugammadex.
This study has several limitations. First, the number of subjects enrolled into the study and number of measurements was small to precisely estimate all model parameters without using external knowledge and Bayesian-based methods. Second, as the model uses prior information, predictions are strongly influenced by the choice of priors, i.e., the results presented by Kleijn et al. [16]. This is especially true for the part of the work that provides large dose extrapolations. Third, we had to discontinue studying patients receiving the smallest dose of sugammadex (0.5 mg/kg), after a paradoxical and life-threatening response of the patient to this dose. Fourth, the simulation does not include the scenarios of sugammadex administration timing based on the TOF ratio.
5 Conclusions
The available rocuronium concentrations, sugammadex concentrations, and TOF ratio data were analyzed using a population Bayesian-based pharmacokinetic–pharmacodynamic model to determine the minimal effective sugammadex dose for different clinical scenarios.
The clinically effective dose of sugammadex should be selected based on the body mass (age) of a child, rocuronium dose, and the time of sugammadex administration after the last rocuronium dose. Assuming the model, the sugammadex dose guaranteeing standard reversal of neuromuscular blockade within 3 min in infants is 1.1 mg/kg (assuming sugammadex administration 20 min post 0.6 mg/kg dose of rocuronium), which should be increased to 1.4, 1.9, 2.6, and 3.2 mg/kg for patients weighing 10, 20, 40, and 70 kg, respectively. The reversal time of 1.5 min requires higher sugammadex doses by a factor of 1.7 (5 kg) to 2 (70 kg), whereas a reversal time of 5 min requires smaller sugammadex doses by a factor of 0.6 (70 kg) to 0.8 (5 kg). As expected, sugammadex doses guaranteeing immediate reversal of neuromuscular blockade within 1.5 min after rocuronium administration at a 1.2 mg/kg rocuronium dose require even higher sugammadex doses ranging from 8.8 to 10.0 mg/kg for infants, and 10.8, 12.2, and 13.1 mg/kg for older children; lower doses than recommended for adults. The proposed PTA analysis suggests that under certain circumstances, a low sugammadex dose might be effective in reversing NMB.
References
Hawkins J, Khanna S, Argalious M. Sugammadex for reversal of neuromuscular blockade: uses and limitations. Curr Pharm Des. 2019;25(19):2140–8.
Honing G, Martini CH, Bom A, van Velzen M, Niesters M, Aarts L, Dahan A, Boon M. Safety of sugammadex for reversal of neuromuscular block. Expert Opin Drug Saf. 2019;18(10):883–91.
Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, Viby-Mogensen J. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology. 2006;104(4):667–74.
Shields M, Giovanelli M, Mirakhur RK, Moppett I, Adams J, Hermens Y. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br J Anaesth. 2006;96(1):36–43.
Suy K, Morias K, Cammu G, Hans P, van Duijnhoven WG, Heeringa M, Demeyer I. Effective reversal of moderate rocuronium- or vecuronium-induced neuromuscular block with sugammadex, a selective relaxant binding agent. Anesthesiology. 2007;106(2):283–8.
Groudine SB, Soto R, Lien C, Drover D, Roberts K. A randomized, dose-finding, phase II study of the selective relaxantbinding drug, sugammadex, capable of safely reversing profound rocuronium-induced neuromuscular block. Anesth Analg. 2007;104(3):555–62.
Plaud B, Meretoja O, Hofmockel R, Raft J, Stoddart PA, van Kuijk JHM, Hermens Y, Mirakhur RK. Reversal of rocuronium-induced neuromuscular blockade with sugammadex in pediatric and adult surgical patients. Anesthesiology. 2009;110(2):284–94.
Sparr HJ, Vermeyen KM, Beaufort AM, Rietbergen H, Proost JH, Saldien V, Velik-Salchner C, Wierda JM. Early reversal of profound rocuronium-induced neuromuscular block by sugammadex in arandomized multicenter study: efficacy, safety, and pharmacokinetics. Anesthesiology. 2007;106(5):935–43.
Gaver RS, Brenn BR, Gartley A, Donahue BS. Retrospective analysis of the safety and efficacy of sugammadex versus neostigmine for the reversal of neuromuscular blockade in children. Anesth Analg. 2019;129(4):1124–9.
Ammar AS, Mahmoud KM, Kasemy ZA. A comparison of sugammadex and neostigmine for reversal of rocuronium-induced neuromuscular blockade in children. Acta Anaesthesiol Scand. 2017;61(4):374–80.
Keating GM. Sugammadex: a review of neuromuscular blockade reversal. Drugs. 2016;76(10):1041–52.
de Boer HD, Carlos RV, Brull SJ. Is lower-dose sugammadex a cost-saving strategy for reversal of deep neuromuscular block? Facts and fiction. BMC Anesthesiol. 2018;18(1):159.
De Cock RFW, Piana C, Krekels EHJ, Danhof M, Allegaert K, Knibbe CAJ. The role of population PK–PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67 Suppl 1(Suppl 1):5–16.
Ploeger BA, Smeets J, Strougo A, Drenth HJ, Ruigt G, Houwing N, Danhof M. Pharmacokinetic–pharmacodynamic model for the reversal of neuromuscular blockade by sugammadex. Anesthesiology. 2009;110(1):95–105.
McDonagh DL, Benedict PE, Kovac AL, Drover DR, Brister NW, Morte JB, Monk TG. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology. 2011;114(2):318–29.
Kleijn HJ, Zollinger DP, van den Heuvel MW, Kerbusch T. Population pharmacokinetic–pharmacodynamic analysisfor sugammadex-mediated reversal of rocuronium-induced neuromuscular blockade. Br J Clin Pharmacol. 2011;72(3):415–33.
Min KC, Lasseter KC, Marbury TC, Wrishko RE, Hanley WD, Wolford DG, de Haes JU, Reitmann C, Gutstein DE. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment. Int J Clin Pharmacol Ther. 2017;55(9):746–52.
Cristinacce A, Wright JG, Macpherson M, Iaconis J, Das S. Comparing probability of target attainment against Staphylococcus aureus for ceftaroline fosamil, vancomycin, daptomycin, linezolid, and ceftriaxone in complicated skin and soft tissue infection using pharmacokinetic/pharmacodynamic models. Diagn Microbiol Infect Di. 2021;99(4): 115292.
Bartkowska-Śniatkowska A, Bienert A, Wiczling P, Owczarek M, Rosada-Kurasińska J, Grześkowiak M, Matysiak J, Kokot ZJ, Kaliszan R, Grześkowiak E. Pharmacokinetics and pharmacodynamics of propofol in children undergoing different types of surgeries. Pharmacol Rep. 2014;66(5):821–9.
Klein AA, Meek T, Allcock E, Cook TM, Mincher N, Morris C, Nimmo AF, Pandit JJ, Pawa A, Rodney G, Sheraton T, Young P. Recommendations for standards of monitoring during anaesthesia and recovery 2021: guideline from the Association of Anaesthetists. Anaesthesia. 2021;76:1212–23.
United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) 2018. Bioanalytical method validation. Guidance for industry. https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Access 15 Feb 2020.
Kwong AHXPC, Calvier EM, Fabre D, Gattacceca F, Khier S. Prior information for population pharmacokinetic and pharmacokinetic/pharmacodynamic analysis: overview and guidance with a focus on the NONMEM PRIOR subroutine. J Pharmacokinet Pharmacodyn. 2020;47(5):431–46.
https://www.ema.europa.eu/en/documents/product-information/bridion-epar-product-information_en.pdf. Accessed 13 Jan 2022.
Tobias JD. Current evidence for the use of sugammadex in children. Pediatr Anesth. 2017;27(7):781.
Paul M, Kindler CH, Fokt RM, Dresser MJ, Dipp NC, Yost CS. The potency of new muscle relaxants on recombinant muscle-type acetylcholine receptors. Anesth Analg. 2002;94(3):597–603.
Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Hunter JM, Sculpher M, Woolacott N. Sugammadex for reversal of neuromuscular block after rapid sequence intubation: a systematic review and economic assessment. Br J Anaesth. 2010;105(5):568–75.
Schaller SJ, Fink H. Sugammadex as a reversal agent for neuromuscular block: an evidence-based review. Core Evid. 2013;8:57–67.
Walas W, Aleksandrowicz D, Borszewska-Kornacka M, Gaszyński T, Helwich E, Migdał M, Piotrowski A, Siejka G, Szczapa T, Bartkowska-Śniatkowska A. Unanticipated difficult airway management in children—the consensus statement of the Paediatric Anaesthesiology and Intensive Care Section and the Airway Management Section of the Polish Society of Anaesthesiology and intensive Therapy and the Polish So. Anaesthesiol Intensive Ther. 2017;49(5):336–49.
Markle A, Horn K, Welter J, Dullenkopf A. An observational study comparing the performance of TOF-Cuff with TOF-Scan monitoring during anaesthetic induction in clinical routine. Anaesthesiol Intensive Ther. 2020;52(3):181–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Support was provided solely from institutional and/or departmental sources.
Conflicts of Interest
The authors declare no competing interests.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences (No 161/17).
Consent to Participate
Written informed consent was obtained from the parents.
Consent for publication
Not applicable.
Availability of Data and Material
Data is provided as electronic supplementary material
Code Availability
The NONMEM code is in the Supplementary Material 2. The Matlab codes used to generate the graphics can be obtained from the corresponding author on reasonable request.
Authors’ Contributions
Participated in clinical study design and study conduct: M.G., A.B., M.M., J.G., K.J., J.R.K., E.G., and A.B.S. Clinical data interpretation: M.G., A.B., and A.B.S. Data curation: J.B. Performed drug concentration measurements: M.K. Performed data analysis and modeling: P.W. All authors contributed to the writing of the manuscript and approved the final version for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Grześkowiak, M., Bienert, A., Wiczling, P. et al. Population Pharmacokinetic–Pharmacodynamic Modeling and Probability of Target Attainment Analysis of Rocuronium and Sugammadex in Children Undergoing Surgery. Eur J Drug Metab Pharmacokinet 48, 101–114 (2023). https://doi.org/10.1007/s13318-022-00809-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00809-1