Abstract
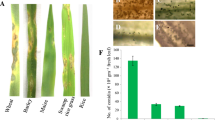
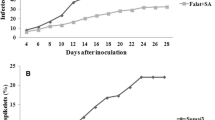
Fusarium head blight (FHB), mainly caused by Fusarium graminearum and F. culmorum, is a worldwide disease of wheat (Triticum aestivum L.), resulting in significant loss in both yield and quality. Use of resistant cultivars is an effective strategy for managing FHB and reducing mycotoxin production in wheat. Understanding of the histochemical, physiological, biochemical and molecular mechanisms involved in FHB resistant and susceptible wheat cultivars is limited so far. In this research, we investigated the role of reactive oxygen species (ROS), non-enzymatic and enzymatic antioxidants in basal resistance of wheat to the hemi-biotrophic and necrotrophic Fusarium species causing FHB. Gaskozhen and Falat plants were used as partially resistant and susceptible wheat cultivars against Fusarium spp., respectively. Accumulation of H2O2 and O2 − was higher in Gaskozhen compared to Falat cultivar. The obtained results revealed considerably higher levels of non-enzymatic and enzymatic antioxidants after inoculation with Fusarium spp. in Gaskozhen compared to Falat cultivar at most of the time points investigated. No significant increase in the accumulation and activity of various antioxidants was observed in the uninoculated control plants in most cases during the time period tested. Significantly higher disease progress in the leaves of both cultivars treated with KCN, as an inhibitor of not only GPOX but also CuZnSOD and MnSOD, revealed the major role of these antioxidantive enzymes compared to FeSOD, APX and CAT in wheat defense responses to Fusarium species. Expression analysis of the genes responsible for production of enzymatic antioxidants using RT-PCR revealed a direct correlation between enzyme activities and expression of the corresponding genes. Application of ROS generating systems increased disease progress in both cultivars. Investigating wheat-Fusarium spp. interaction showed that F. culmorum isolate induced higher levels of ROS and antioxidants compared to F. graminearum. In addition, application of enzymatic antioxidant inhibitors reduced H2O2 and O2 − levels, which led to increased disease development.








Similar content being viewed by others
References
Able AJ (2003) Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 221(1–2):137–143
Adhikari TB, Balaji B, Breeden J, Goodwin SB (2007) Resistance of wheat to Mycosphaerella graminicola involves early and late peaks of gene expression. Physiol Mol Plant Pathol 71:55–68
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Amarasinghe CC, Tamburic-Ilincic L, Gilbert J, Brûlé-Babel AL, Fernando WGD (2013) Evaluation of different fungicides for control of fusarium head blight in wheat inoculated with 3ADON and 15ADON chemotypes of Fusarium graminearum in Canada. Can J Plant Pathol 35:200–208
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Atanasova-Penichon V, Barreau C, Richard-Forget F (2016) antioxidant secondary metabolites in cereals: ootential involvement in resistance to fusarium and mycotoxin accumulation. Front Microbiol 7:566
Baek KH, Skinner DZ (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci 165:1221–1227
Barna B, Fodor J, Harrach BD, Pogány M, Király Z (2012) The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol Biochem 59:37–43
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bennett M, Mehta M, Grant M (2005) Biophoton imaging: a nondestructive method for assaying R gene responses. Mol Plant-Microbe Interact 18:95–102
Boba A, Kulma A, Kostyn K, Starzycki M, Starzycka E, Szopa J (2011) The influence of carotenoid biosynthesis modification on the Fusarium culmorum and Fusarium oxysporum resistance in flax. Physiol Mol Plant Pathol 76:39–47
Bollina V, Kumaraswamy GK, Kushalappa AC, Choo TM, Dion Y, Rioux S, Faubert D, Hamzehzarghani H (2010) Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against fusarium head blight. Mol Plant Pathol 11:769–782
Boutigny AL, Ward T, Ballois N, Iancu G, Ioos R (2014) Diversity of the Fusarium graminearum species complex on French cereals. Eur J Plant Pathol 138:133–148
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brewer HC, Hawkins ND, Hammond-Kosack KE (2014) Mutations in the Arabidopsis homoserine kinase gene DMR1 confer enhanced resistance to Fusarium culmorum and F. graminearum. Brewer et al. BMC Plant Biology 14:317
Browne RA, Cooke BM (2004) Development and evaluation of an in vitro detached leaf assay for pre-screening resistance to fusarium head blight in wheat. Eur J Plant Pathol 110:91–102
Bushnell WR, Hazen BE, Pritsch C (2003) Histology and physiology of fusarium head blight. In: Kurt JL, Bushnell WR (eds) Fusarium head blight of wheat and barley. APS Press, St. Paul, MN, pp. 44–83
Chen, Y. J., Lyngkj,M. F.,&Collinge, D. B. (2013). Future prospects for genetically engineering disease-resistant plants. In molecular plant immunity, G Sessa, Ed, Wiley-Blackwell
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Ecol Environ 2:53
De Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–97
De Vleesschauwer D, Chernin L, Höfte M (2009) Differential effectiveness of Serratia plymuthica IC1270-induced systemic resistance against hemibiotrophic and necrotrophic leaf pathogens in rice. BMC Plant Biol 9:1–16
Debona D, Rodrigues FÁ, Rios JA, Nascimento KJT (2012) Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 102:1121–1129
Desmond OJ, Manners JM, Stephens AE, MacLean DJ, Schenk PM, Gardiner DM, Munn AL, Kazan K (2008) The fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol Plant Pathol 9:435–445
Ding L, Xu H, Yi H, Yang L, Kong Z, Zhang L, Xue S, Jia H, Ma Z (2011) Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE. 6:e19008
Dong CH, Zolman BK, Bartel B, Lee B, Stevenson B, Agarwal B, Zhu JK (2009) Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol Plant 2:59–72
Durner J, Klessig DF (1995) Inhibition of ascorbate peroxidase by salicylic acid and 2, 6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci U S A 92:11312–11316
Elstner EF, Heupel A (1976) Formation of hydrogen peroxide by isolated cell walls from horseradish. Planta 130:175–180
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gao C, Zhang L, Wen F, Xing D (2008) Sorting out the role of reactive oxygen species during plant programmed cell death induced by ultraviolet-C overexposure. Plant Signal Behav 3(3):197–198
Gapinska M, Sklodowska M, Gabara B (2008) Effect of short- and long-termsalinity on the activities of antioxidative enzymes and lipid peroxidation in tomatoroots. Acta Physiol Plant 30:11–18
Gherbawy YA, El-Tayeb MA, Maghraby TA, Shebany YM, El-Deeb BA (2012) Response of antioxidant enzymes and some metabolic activities in wheat to Fusarium spp. infections. Acta Agron Hung 60:319–333
Gilbert J, Tekauz A (2011) Strategies for management of fusarium head blight (FHB) in cereals. Prairie Soils and Crops 4:97–104
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gomathi R, Rakkiyapan P (2011) Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int J Plant Physiol Biochem 3:67–74
Gosman N, Srinivasachary A, Steed E, Chandler E, Thomsett M, Nicholson P (2010) Evaluation of type I fusarium head blight resistance of wheat using non-deoxynivalenol-producing fungi. Plant Pathol 59:147–157
Goswami RS, Kistler HC (2005) Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology 95:1397–1404
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Gunnaiah R, Kushalappa AC (2014) Metabolomics deciphers the host resistance mechanisms in wheat cultivar Sumai-3, against trichothecene producing and non-producing isolates of Fusarium graminearum. Plant Physiol Biochem 83:40–50
Gunnaiah R, Kushalappa AC, Duggavathi R, Fox S, Somers DJ (2012) Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL (Fhb1) contributes to resistance against Fusarium graminearum. PLoS One 7:e40695
Guo Z, Tan H, Zhu Z, Lu S, Zhou B (2005) Effect of intermediates on ascorbic acid and oxalate biosynthesis of rice and in relation to its stress resistance. Plant Physiol Biochem 43:955–962
Gutierrez-Gonzalez JJ, Wise ML, Garvin DF (2013) A developmental profile of tocol accumulation in oat seeds. J Cereal Sci 57:79–83
Harborne JB (1973) Phytochemical methods. Chapman and Hall Ltd, London, pp. 49–188
Havaux M (2013) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Ji X, Dong B, Shiran B, Talbot MJ, Edlington JE, White RG, Gubler F, Dolferus R (2011) Control of abscisic acid catabolism and abscisic acid homoeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol 156:617–662
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 162750:1–16
Li G, Yen Y (2008) Jasmonate and ethylene signaling pathway may mediate fusarium head blight resistance in wheat. Crop Sci 48:1888–1896
Li A, Wang X, Leseberg CH, Jia J, Mao L (2008a) Biotic and abiotic stress responses through calcium-dependent protein kinase (CDPK) signaling in wheat (Triticum aestivum L.). Plant Signal Behav 3:654–656
Li C, Barker SJ, Gilchrist DG, Lincoln JE, Cowling WA (2008b) Leptosphaeria maculans elicits apoptosis coincident with leaf lesion formation and hyphal advance in Brassica napus. Mol Plant-Microbe Interact 21:1143–1153
Lightfoot DJ, Mcgrann GR, Able AJ (2016) The role of a cytosolic superoxide dismutase in barley-pathogen interactions. Mol Plant Pathol. doi:10.1111/mpp.12399
Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O2 −, H2O2, and OH−) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
Mesterhazy A (1995) Types and components of resistance to fusarium head blight of wheat. Plant Breed 114:377–386
Mesterhazy A, Toth B, Bartok T, Varga M (2008) Breeding strategies against FHB in winter wheat and their relation to TypeI resistance. Cereal Res Commun 36:37–43
Miedaner T, Schneider B, Geiger HH (2003) Deoxynivalenol (DON) content and fusarium head blight resistance insegregating populations of winter rye and winter wheat. Crop Sci 43:519–526
Mika A, Lüthje S (2003) Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiol 132:1489–1498
Mohammadi M, Kazemi H (2002) Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci 162:491–498
Morita S, Kaminaka H, Masumura T, Tanaka K (1999) Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress: the involvement of hydrogen peroxide in oxidative stress signaling. Plant Cell Physiol 40:417–422
Motallebi P, Niknam V, Ebrahimzadeh H, Hashemi M, Pisi A, Prodi A, Tonti S, Nipoti P (2015a) Methyl jasmonate strengthens wheat plants against root and crown rot pathogen fusarium culmorum infection. J Plant Growth Regul 34(3):624–636
Motallebi P, Niknam V, Ebrahimzadeh H, Tahmasebi Enferadi S, Hashemi M (2015b) The effect of methyl jasmonate on enzyme activities in wheat genotypes infected by the crown and root rot pathogen Fusarium culmorum. Acta Physiol Plant 37:237
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidases in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nikraftar F, Taheri P, Flahati-Rastegar M, Tarighi S (2013) Tomato partial resistance to Rhizoctonia solani involves antioxidative defense mechanisms. Physiol Mol Plant Pathol 81:74–83
Noorbakhsh Z, Taheri P (2016) Nitric oxide: a signaling molecule which activates cell wall-associated defense of tomato against Rhizoctonia solani. Eur J Plant Pathol 144:551–568
Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84:663–679
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6:69
Pettersson H, Aberg L (2003) Near infrared spectroscopy for determination of mycotoxins in cereals. Food Cont 14:229–232
Petti C, Reiber K, Ali SS, Berney M, Doohan FM (2012) Auxin as a player in the biocontrol of Fusarium head blight of barley and its potential as a disease control agent. BMC Plant Biol 12:224
Ponts N, Pinson-Gadais L, Barreau C, Richard-Forget F, Ouellet T (2007) Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Lett 581:443–447
Ponts N, Couedelo L, Pinson-Gadais L, Verdal-Bonnin MN, Barreau C, Richard-Forget F (2009) Fusarium response to oxidative stress by H2O2 is trichothecene chemotype-dependent. FEMS Microbiol Lett 293:255–262
Ravensdale M, Rocheleau H, Wang L, Nasmith C, Ouellet T, Subramaniam R (2014) Components of priming-induced resistance to fusarium head blight in wheat revealed by two distinct mutants of Fusarium graminearum. Mol Plant Pathol 15:948–956
Reddy BV, Boyadjieva N, Sarkar DK (1995) Effect of ethanol, propanol, butanol, and catalase enzyme blockers on β-endorphin secretion from primary cultures of hypothalamic neurons: evidence for a mediatory role of acetaldehyde in ethanol stimulation of β-endorphin release. Alcohol Clin Exp Res 19:339–344
Samalova M, Meyer AJ, Gurr SJ, Fricker MD (2014) Robust anti-oxidant defences in the rice blast fungus Magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytol 201:556–573
Scherm B, Balmas V, Spanu F, Pani G, Delogu G, Pasquali M, Migheli Q (2013) Fusarium culmorum: causal agent of foot and root rot and head blight on wheat. Mol Plant Pathol 14:323–341
Schroeder HW, Christensen JJ (1963) Factors affecting resistance of wheatto scab by Gibberellazeae. Phytopathology 53:831–838
Shetty NP, Kristensen BK, Newman MA, Møller K, Gregersen PL, Jørgensen HJL (2003) Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol Mol Plant Pathol 62:333–346
Shetty NP, Mehrabi R, Lütken H, Haldrup A, Kema GHJ, David B, Collinge DB, Jorgensen HJL (2007) Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol 174:637–664
Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS (2008) Roles of reactive oxygen species in interactions between plants and pathogens. Eur J Plant Pathol 121:267–280
Siddhuraju P, Becker K (2003) Antioxidant properties of various extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera L.) leaves. J Agric Food Chem 51:2144–2155
Soltanloo H, Ghadirzade Khorzoghi E, Ramezanpour SS, Kalateh Arabi M (2011) Genetic analysis of fusarium head blight resistance in bread wheat. Australas Plant Pathol 40:453–460
Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H (2015a) Differential antioxidative responses of susceptible and resistant wheat cultivars against fusarium head blight. Int J Farm and Alli Sci 4:239–243
Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Behmanesh M, Tahmasebi Enferadi S (2015b) Central role of salicylic acid in resistance of wheat against Fusarium graminearum. J Plant Growth Regul:1–15
Sorahinobar M, Niknam V, Ebrahimzadeh H, Soltanloo H, Moradi B, Bahram M (2015c) Lack of association between Fusarium graminearum resistance in spike and crude extract tolerance in seedling of wheat. Eur J Plant Pathol 144:525–538
Taheri P, Tarighi S (2010) Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J Plant Physiol 167:201–208
Taheri P, Tarighi S (2011) A survey on basal resistance and riboflavin-induced defense responnses of sugar beet against Rizoctonia solani. J Plant Physiol 168:1114–1122
Taheri P, Irannejad A, Goldani M, Tarighi S (2014) Oxidative burst and enzymatic antioxidant systems in rice plants during interaction with Alternaria alternate. Eur J Plant Pathol 140:829–839
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Urbanek H, Kuzniak-Gebarowska E, Herka K (1991) Elicitation of defense responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol Plant 13:43–50
Velikova V, Yordanov L, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Walter S, Nicholson P, Doohan FM (2010) Action and reaction of host and pathogen during fusarium head blight disease. New Phytol 185:54–66
Xu FJ, Jin CW, Liu WJ, Zhang YS, Lin XY (2011) Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J Integr Plant Biol 53:44e53
Yoshida M, Kawada N, Nakajima T (2007) Effect of infection timing on fusarium head blight and mycotoxin accumulation in open and closed-flowering barley. Phytopathology 97:1054–1062
Yu Q, Rengel Z (1999) Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupines. Ann Bot 83:175–182
Zhang X, Fu J, Hiromasa Y, Pan H, Bai G (2013a) Differentially expressed proteins associated with fusarium head blight resistance in wheat. PLoS One 8(12):e82079
Zhang P, Zhou MP, Zhang X, Huo Y, Ma HX (2013b) Change of defensive-related enzyme in wheat crown rot seedlings infected by Fusarium graminearum. Cereal Res Commun 41:431–439
Zhang ZQ, Xiang JJ, Zhou LM (2015) Antioxidant activity of three components of wheat leaves: ferulic acid, flavonoids and ascorbic acid. J Food Sci Technol 52:7297–7304
Zhao L, He JX, Wang XM, Zhang LX (2008) Nitric oxide protects against polyethylene glycol-induced oxidative damage in two ecotypes of reed suspension cultures. J Plant Physiol 165:182–191
Zhou W, Kolb FL, Riechers DE (2005) Identification of proteins induced or upregulated by fusarium head blight infection in the spikes of hexaploid wheat (Triticum aestivum). Genome 48:770–780
Zhou K, Hao J, Griffey C, Chung H, O'Keefe SF, Chen J, Hogan S (2007) Antioxidant properties of fusarium head blight-resistant and -susceptible soft red winter wheat grains grown in Virginia. J Agric Food Chem 55:3729–3736
Acknowledgements
We thank Ferdowsi University of Mashhad, Iran, for financial support of this research with project number 31477 approved on 2/07/2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khaledi, N., Taheri, P. & Falahati-Rastegar, M. Reactive oxygen species and antioxidant system responses in wheat cultivars during interaction with Fusarium species. Australasian Plant Pathol. 45, 653–670 (2016). https://doi.org/10.1007/s13313-016-0455-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0455-y