Abstract
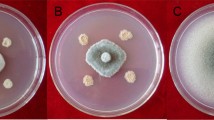
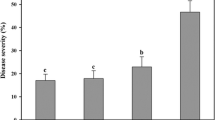
Biological control, especially with Bacillus-based biocontrol agents, offers an attractive alternative to synthetic pesticides for sustainable management of white mold disease caused by Sclerotinia sclerotiorum. In this study, eight effective Bacillus isolates were isolated from rhizospheric soil samples as potential bacterial biocontrol agents. Cultural, biochemical, and molecular analyses of 16S rDNA and gyrase subunit A (gyrA) confirmed that all isolates were identified as Bacillus amyloliquefaciens subsp. plantarum. The production of hydrolytic enzymes and the plant growth-promotional attributes of these isolates confirmed their multifaceted potential. Molecular analysis of the eight biosynthetic genes, which are related to antibiotic properties of bacilli, revealed that all of the isolates possess five genes: bacA for bacilysin, dfnM for difficidin, fenA for fengycin, ituA for iturin, and sfp for surfactin. The Bacillus isolates inhibited mycelial growth and suppressed formation of sclerotia during an in vitro test against S. sclerotiorum. Deformities and cell-wall lysis of mycelia, abnormalities of apothecia, and germination failure of ascospores through interaction with the Bacillus isolates were observed with light and scanning electron microscopes, suggesting that they have high antagonistic effects against S. sclerotiorum. Seed bacterization with the Bacillus isolates protected mustard seedlings in vitro up to 98 % against S. sclerotiorum. In a pot experiment, damages of mustard plants against the pathogen decreased up to 90 % after foliar spray of the Bacillus isolates. In addition, the isolates increased seed germination and accelerated seedling vigor of mustard, suggesting that they have plant growth-promoting functions.







Similar content being viewed by others
References
Abdul-Baki AA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Abdullah MT, Ali NY, Suleman P (2008) Biological control of Sclerotinia sclerotiorum (Lib.) de bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Prot 27:1354–1359
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P (2009) Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Factories 8(63). doi:10.1186/1475-2859-8-63
Ashwini N, Srividya S (2013) Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. doi:10.1007/s13205-013-0134-4
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16
Borriss R, Chen XH, Rueckert C, Blom J, Becker A, Baumgarth B, Fan B, Pukall R, Schumann P, Spröer C, Junge H, Vater J, Pühler A, Klenk HP (2011) Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int J Syst Evol Microbiol 61:1786–1801
Cazorla FM, Romero D, Pérez-García A, Lugtenberg BJJ, de Vicente A, Bloemberg G (2007) Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J Appl Microbiol 103:1950–1959
Chaurasia B, Pandey A, Palni LMS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Süssmuth R, Piel J, Borriss R (2009a) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
Chen XH, Scholz R, Borriss M, Junge H, Mögel G, Kunz S, Borriss R (2009b) Difficidin and bacilysin produced by plant-associated Bacillus amyloliquefaciens are efficient in controlling fire blight disease. J Biotechnol 140:38–44
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp. and plants with special reference to induced systemic resistance (ISR). Microbiol Res 164:493–513
Chun J, Bae KS (2000) Phylogenetic analysis of Bacillus subtilis and related taxa based on partial gyrA gene sequences. Antonie Van Leeuwenhoek 78:123–127
Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F (1997) Biological control of Pythium ultimum by Stenotrophomonas maltophilia W81 is mediated by an extracellular proteolytic activity. Microbiol 143:3921–3931
Gerlagh M, Goossen-van d GHM, Fokkema NJ, Vereijken PFG (1999) Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology 89:141–147
Hou X, Boyetchko SM, Brkic M, Olson D, Ross A, Hegedus D (2006) Characterization of the anti-fungal activity of a Bacillus spp. associated with sclerotia from Sclerotinia sclerotiorum. Appl Microbiol Biotechnol 72:644–653
Hu X, Roberts DP, Jiang M, Zhang Y (2005) Decreased incidence of disease caused by Sclerotinia sclerotiorum and improved plant vigor of oilseed rape with Bacillus subtilis Tu-100. Appl Microbiol Biotechnol 68:802–807
Idris HA, Labuschagne N, Korsten L (2007) Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol Control 40:97–106
Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, Romeo T (2002) Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol 184:290–301
Kloepper JW, Ryu C-M, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Kumar P, Dubey RC, Maheshwari DK (2012) Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol Res 167:493–499
Leelasuphakul W, Sivanunsakul P, Phongpaichit S (2006) Purification, characterization and synergistic activity of β-1,3-glucanase and antibiotic extract from an antagonistic Bacillus subtilis NSRS 89-24 against rice blast and sheath blight. Enzym Microb Technol 38:990–997
McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology 94:1252–1258
Minaxi LN, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135
Mueller DS, Dorrance AE, Derksen RC, Ozkan E, Kurle JE, Grau CR, Gaska JM, Hartman GL, Bradley CA, Pedersen WL (2002) Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis 86:26–31
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Pérez-García A, Romero D, de Vicente A (2011) Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr Opin Biotechnol 22:187–193
Perneel M, Heyrman J, Adiobo A, De Maeyer K, Raaijmakers JM, De Vos P, Höfte M (2007) Characterization of CMR5c and CMR12a, novel fluorescent Pseudomonas strains from the cocoyam rhizosphere with biocontrol activity. J Appl Microbiol 103:1007–1020
Purdy LH (1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology 69:875–880
Rahman MME, Dey TK, Hossain DM, Nonaka M, Harada N (2015) First report of white mold caused by Sclerotinia sclerotiorum on jackfruit. Australasian Plant Dis Notes 10:10. doi:10.1007/s13314-014-0155-9
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051–1056
Sharma N, Sharma S (2008) Control of foliar diseases of mustard by Bacillus from reclaimed soil. Microbiol Res 163:408–413
Shi J, Li Y, Qian H, Du G, Chen J (2004) Pre-germinated conidia of Coniothyrium minitans enhances the foliar biological control of Sclerotinia sclerotiorum. Biotechnol Lett 26:1649–1652
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Souto GI, Correa OS, Montecchia MS, Kerber NL, Pucheu NL, Bachur M (2004) Genetic and functional characterization of a Bacillus sp. strain excreting surfactin and antifungal metabolites partially identified as iturin-like compounds. J Appl Microbiol 97:1247–1256
Steadman JR (1979) Control of plant diseases caused by Sclerotinia species. Phytopathology 69:904–907
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52:487–511
Yang D, Wang B, Wang J, Chen Y, Zhou M (2009) Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and sclerotinia stem rot of rape. Biol Control 51:61–65
Yoshida S, Hiradate S, Tsukamot T, Hatakeda K, Shirata A (2001) Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 91:181–187
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturinA by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963
Zhang JX, Xue AG (2010) Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathol 59:382–391
Zhao P, Quan C, Wang Y, Wang J, Fan S (2014) Bacillus amyloliquefaciens Q-426 as a potential biocontrol agent against Fusarium oxysporum f. sp. spinaciae. J Basic Microbiol 54:448–456
Zheng XY, Sinclair JB (2000) The effects of traits of Bacillus megaterium on seed and root colonization and their correlation with the suppression of Rhizoctonia root rot of soybean. BioControl 45:223–243
Acknowledgments
The authors wish to thank Dr. Guan and Mr. Shoji, Niigata University, Japan, for assisting with the collection of soil samples for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest regarding the publication of this article.
Rights and permissions
About this article
Cite this article
Rahman, M.M., Hossain, D.M., Suzuki, K. et al. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Australasian Plant Pathol. 45, 103–117 (2016). https://doi.org/10.1007/s13313-016-0397-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-016-0397-4