Abstract
Objective
To study the etiology of neuroregression in children having deficiency of the lysosomal enzymes.
Design
Review of medical records.
Setting
Specialized Genetic Center.
Participants
432 children aged 3 mo-18 y having regression in a learned skill, selected from 1453 patients referred for diagnostic workup of various Lysosomal storage disorders (LSDs).
Methods
Plasma chitotriosidase, quantitative and qualitative glycosaminoglycans, and mucolipidosis-II/II screening followed by confirmatory enzyme study using specific substrate was carried out; Niemann-Pick disease Type-C was studied by fillipin stain method on skin fibroblasts.
Results
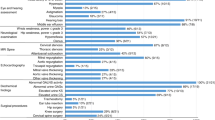
Total 309 children (71.5%) were diagnosed with different lysosomal storage disorders as the underlying cause of neuroregression. Plasma chitotriosidase was raised in 82 of 135; 64 (78%) of these had various LSDs. 69 out of 90 cases showed high excretion of glycoaminoglycans, and 67 (97.1%) of these were confirmed to have enzyme deficiency for various mucoplysaccharide disorders. While 3/90 children with positive I-cell screening had confirmed mucolipidosis-II/III disease. Among all, glycolipid storage disorders were the most common (50.2%) followed by mucopolysaccharidosis (MPS) (21.7%) and sulphatide degradation defect (17.5%). Neuronal ceroid lipofucinosis-1 & 2 (7.4%), mucolipidosis-II/III (1%), Sialic acid storage disorder (1%), Niemann-Pick disease type-C (1%) and Fucosidosis (0.3%) were observed with less frequency. Most common phenotypes in all subjects were cherry red spot (18.5%), hepatosplenomegaly (17.9%), coarse facies (15%), seizures (13.1%) and skeletal abnormalities (12.14%).
Conclusions
Lysosomal storage disorders are considered to be one of the common causes in children with regression in learned skill, dysmorphic features and cherry red spot. Among these, glycolipid storage disorders are the most common, followed by mucopolysaccharidosis.
Similar content being viewed by others
References
Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21:65–75.
Tomas, Vila M, Vitoria MI, Gomez GF, Pantoja MJ, Revert GM, et al. Epidemiology of progressive intellectual and neurological deterioration in childhood-A multicentre study in the Community of Valencia. Anales de Pediatría. 2013;78:303–07.
Verity CM, Winstone AM, Stellitano L, Krishnakumar D, Will R, Mcfarland. The clinical presentation of mitochondrial diseases in children with progressive intellectual and neurological deterioration: a national, prospective, population-based study. Dev Med Child Neurol. 2009;52:434–40.
Agrawal S, Nathani S. Neuroregression in vitamin B12 deficiency. BMJ case reports 2009: bcr0620080235. doi:10.1136/bcr.06.2008.0235.
Jain S, Chowdhury V, Juneja M, Kabra M, Pandey S, Singh A, et al. Intellectual disability in Indian children: Experience with a stratified approach for etiological diagnosis. Indian Pediatr. 2013;50:1125–30.
Vellodi A. Lysosomal storage disorders. Br J Haematol. 2005;128:413–31.
Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–65.
Wilcox WR. Lysosomal storage disorders: the need for better pediatric recognition and comprehensive care. J Pediatr. 2004;144:3–14.
Verity C, Winstone AM, Stellitano L, Will R, Nicoll A. The epidemiology of progressive intellectual and neurological deterioration in childhood. Arch Dis Child. 2010;95: 361–4.
Verma PK, Ranganath P, Dalal AB, Phadke SR. Spectrum of Lysosomal storage disorders at a medical genetics center in northern India. Indian Pediatr. 2012; 49:799–804.
Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–54.
Meikle PJ, Ranieri E, Simonsen H, Rozaklis T, Ramsay SL, Whitfield PD, et al. Newborn screening for lysosomal storage disorders: clinical evaluation of a two-tier strategy. Pediatrics. 2004;114:909–16.
Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, Van WS, et al. The frequency of lysosomal storage disease in the Netherlands. Human Genet. 1999;105:151–6.
Poupetova H, Ledvinova J, Berna L, Dvorakova L, Kozich V, Elleder M. The birth prevalence of lysosomal storage disorders in the Czech Republic: comparison with data in different populations. J Inherit Metab Dis. 2010;33: 387–96.
Lake BD, Young EP, Winchester BG. Prenatal diagnosis of lysosomal storage diseases. Brain Pathol. 1998;8: 133–49.
Sheth J, Mistri M, Sheth F, Datar C, Godbole K, Kamate M, et al. Prenatal diagnosis of lysosomal storage disorders by enzymes study using chorionic villus and amniotic fluid. J Fetal Med. 2014;1:17–24.
Mechtler TP, Metz TF, Muller HG, Ostermann K, Ratschmann R, De Jesus VR, et al. Short-incubation mass spectrometry assay for lysosomal storage disorders in newborn and high-risk population screening. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;908:9–17.
Hoffmann B, Mayatepek E. Neurological manifestations in lysosomal staorage disorders–from pathology to first therapeutic options. Neuropediatrics. 2005;36:285–9.
Wang RY, Bodamer OA, Watson MS, Wilcox WR. Lysosomal storage diseases: Diagnostic confirmation and management of presymptomatic individuals. Genet Med. 2011;13:457–84.
Sheth J, Patel P, Sheth F, Shah R. Lysosomal storage disorders. Indian Pediatr. 2004;41:260–5.
Sheth J, Sheth F, Oza N, Gambhir P, Dave U, Shah R. Plasma chitotriosidase activity in children with lysosomal storage disorders. Indian J Pediatr. 2010;77:203–5.
Sheth J, Mistri M, Kamate M, Vaja S, Sheth FJ. Diagnostic strategy for Mucolipidosis II/III. Indian Pediatr. 2012;49:975–7.
Sheth J, Mistri M, Sheth F, Shah R, Bavdekar A, Godbole K, et al. Burden of lysosomal storage disorders in India: experience of 387 affected children from a single diagnostic facility. JIMD Rep. 2014;12:51–63.
Dembure, Philip P, Roesel AR. Screening for mucopolysaccharidoses by analysis of urinary glycosaminoglycans. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. Wiley-Liss, New York. 1991. p.77–86.
Shapria E, Blitzer MG, Miller JB, Africk DK. Fluorometric assays in biochemical genetics: a laboratory Manual. New York, NY: Oxford University Press. 1989. p. 19–46.
Kruth, Howard S, Vaughan M. Quantification of low density lipoprotein binding and cholesterol accumulation by single human fibroblasts using fluorescence microscopy. J Lipid Res. 1980;21:123–30.
Sheth J, Sheth F, Oza N. Niemann-pick type C disease. Indian Pediatr. 2008;45:505–7.
Mistri M, Tamhankar PM, Sheth F, Sanghavi D, Kondurkar P, Patil S, et al. Identification of novel mutations in HEXA gene in children affected with Tay Sachs disease from India. PLoS One. 2012;7:e39122.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheth, J., Mistri, M., Bhavsar, R. et al. Lysosomal storage disorders in Indian children with neuroregression attending a genetic center. Indian Pediatr 52, 1029–1033 (2015). https://doi.org/10.1007/s13312-015-0768-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-015-0768-x