Abstract
This study explored the efficacy and safety of transcutaneous auricular vagus nerve stimulation (ta-VNS) in patients with epilepsy. A total of 150 patients were randomly divided into active stimulation group and control group. At baseline and 4, 12, and 20 weeks of stimulation, demographic information, seizure frequency, and adverse events were recorded; at 20 weeks, the patients underwent assessment of quality of life, Hamilton Anxiety and Depression scale, MINI suicide scale, and MoCA scale. Seizure frequency was determined according to the patient’s seizure diary. Seizure frequency reduction > 50% was considered effective. During our study, the antiepileptic drugs were maintained at a constant level in all subjects. At 20 weeks, the responder rate was significantly higher in active group than in control group. The relative reduction of seizure frequency in the active group was significantly higher than that in the control group at 20 weeks. Additionally, no significant differences were shown in QOL, HAMA, HAMD, MINI, and MoCA score at 20 weeks. The main adverse events were pain, sleep disturbance, flu-like symptoms, and local skin discomfort. No severe adverse events were reported in active and control group. There were no significant differences in adverse events and severe adverse events between the two groups. The present study showed that ta-VNS is an effective and safe therapy for epilepsy. Furthermore, the benefit in QOL, mood, and cognitive state of ta-VNS needs further validation in the future study although no significant improvement was shown in this study.
Similar content being viewed by others
Introduction
Epilepsy is a common chronic disease of central nervous system, which is featured by recurrent unprovoked seizures, affecting about 50 million people worldwide according to the report by the World Health Organization (WHO) [1, 2]. However, approximately one third of the patients are not able to achieve seizure freedom despite appropriate medications and advent of new anti-seizure medications (ASMs), which is known as drug-resistant epilepsy [3]. For these patients, alternative treatment options include resective neurosurgery and neuromodulation such as deep brain stimulation and vagus nerve stimulation (VNS) [4]. Among all neuromodulation treatments, invasive vagus nerve stimulation (i-VNS) is most frequently used, which requires a stimulator and an electrode to be implanted and connected to the vagus nerve. In previous clinical trials, invasive stimulation of the cervical branch of the vagus nerve has been recognized to be effective with a responder rate of about 40–60% [5, 6].
However, the many side effects associated with i-VNS cannot be ignored. First, the implantation of the stimulator and electrode is an invasive surgery procedure which requires general anesthesia, bringing operation- and anesthesia-associated risk to the patients [7, 8]. Second, i-VNS could result in various surgically and technically induced postoperative complications, such as hoarseness, cough, pain, deep wound infections, cardiac arrhythmia, and device fracture or malfunction [9, 10]. Thus, there is a medical demand for an alternative VNS device which is more selective and noninvasive to the patients with a lower risk.
Transcutaneous auricular vagus nerve stimulation (ta-VNS) is a newly developed treatment which could overcome the drawbacks of i-VNS [11]. The auricular branch of the vagus nerve, which supplies the cymba conchae, could be stimulated using an external device with a bipolar electrode attached to the skin of the left ear conch [12]. Pilot studies of ta-VNS on epilepsy suggested responder rate (seizure reduction ≥ 50%) and mean seizure reduction could reach up to 53.85% and 54.21% respectively [13, 14]. In subsequent studies, Bauer et al. suggested a responder rates (seizure reduction ≥ 50%) of 27.0% and 25.6% in high-frequency and low-frequency stimulation groups respectively [15]; some other studies showed the mean seizure frequency reduction by ta-VNS treatment could reach to about 40% [15,16,17].
The present study was a randomized, double-blinded, controlled trial investigating the efficacy and safety of ta-VNS as a treatment option for epilepsy. The main objective of this study was to demonstrate superiority of add-on therapy with 20 weeks of ta-VNS (active stimulation) versus control in reducing seizure frequency and to investigate the influence of ta-VNS on the quality of life, mood, and cognitive status.
Materials and Method
Patients
The patients in the present study were enrolled from March 2019 to February 2021 at 4 centers (Capital Medical University affiliated Beijing Tiantan Hospital, Nanjing Medical University affiliated Brain Hospital, Tianjin Medical University General Hospital, and The First Affiliated Hospital of Zhengzhou University). The study period per patient was 28 weeks. The whole study was designed, implemented, and reported following ISO 14155, with applicable local laws and regulations, and with the ethical principles laid down in the Declaration of Helsinki and described in the ICH-GCP guidelines. Approvals from all responsible ethic committees were obtained (Aug. 8, 2017, QX 2017–005-02), prior to the initiation of this study.
All patients or their guardians provided written informed consent. The inclusion criteria were as follows: (1) age 18–65 years old (including 18 and 65 years old); (2) the patients met the ILAE definition of epilepsy [18], taking 2 or more types of ASMs over 2 years with ineffective seizure control (the average seizure frequency in recent 1 year was ≥ 4 times per month, and ≥ 1 time every month), and deemed not suitable for surgery or unwilling to take the risk of surgery; (3) the dose of the applied antiepileptic drugs (ASMs) did not change 4 weeks before the baseline period and throughout the period of the study. The exclusion criteria included (1) status epilepticus occurred in recent 1 year; (2) progressive brain diseases; (3) serious somatic diseases such as heart, liver, and kidney diseases (ECG indicates severe myocardial ischemia or arrhythmia; one of ALT, AST, BUN, and Cr in blood biochemistry test is ≥ 2 times of the normal level) or serious neuropsychiatric diseases; (4) history of alcohol addiction or drug abuse; (5) females who are pregnant or planning pregnancy; (6) equipped with cardiac pacemaker, vagus nerve stimulator, or metal implant; and (7) patients with peptic ulcer.
Study Design and Treatment
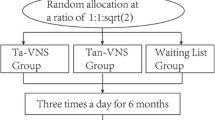
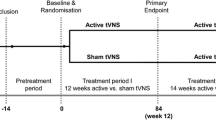
A total of 150 patients were enrolled in this study. The number of patients was determined by a power analysis. During an 8-week baseline period, an interview was used to record general patient information including age, gender, duration of epilepsy, type of epilepsy, family history, the number and dosage of ASMs, interictal EEG, and MRI. Baseline seizure frequency was documented in a patient diary. Following the 8-week baseline period, the patients were randomized into 2 groups (100 patients in active stimulation group and 50 patients in control group). The questionnaires investigating the patient’s quality of life (QOL), mood, and cognitive state at baseline included QOLIE-31, Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), Mini-International Neuropsychiatric Interview (MINI) suicide risk scale, and Montreal Cognitive Assessment (MoCA) (Figs. 1 and 2).
At the beginning of the 20-week treatment period, patients were randomized to two group: the active group (n = 100) received active stimulation with 25-Hz stimulation frequency, 250-µs pulse width, and 30-s on/30-s off, over a period of 2 h per day divided into 4 periods of 30 min; the stimulation was maintained at a stable level that maximum tolerated intensity of pain was felt by the patients. The control group (n = 50) received apparent same period of treatment but the stimulation frequency was 1 Hz and the intensity was maintained at a stable level that minimum perceptible tingling was felt which made the patients believe they were receiving active stimulation while they were actually not. Stimulation was performed using the NIFDC certified t-VNS device TVNS-100 (Xinzhile, Jiangxi, China) (Fig. 3). The patients’ current ASM treatment was not changed during the study. Three treatment visits (weeks 4, 12, 20) were performed. Seizure frequency was prospectively recorded in patient diaries. Adverse events during the treatment were reported by patients. Vital signs, physical examination, electrocardiogram (ECG), and assessment of QOL, mood, and cognitive state were performed at the above time points.
Study Outcomes
Primary efficacy variable was the responder rate at 20 weeks of treatment, which is defined as percentage of patients who achieved ≥ 50% reduction in mean seizure frequency from baseline to the end of treatment. Secondary efficacy variables comprised reduction in seizure frequency from baseline to each treatment visit (4, 12, 20 weeks) and changes in QOLIE-31, HAMA, HAMD, MINI suicide risk scale, and MoCA. Safety was primarily assessed by analysis of non-severe and severe adverse events (AEs) and device-related AEs which emerged during treatment and reported by patients. Additional safety measurements included vital signs, physical examination, and 12-lead ECG.
Statistical Analysis
All summary statistics are presented by groups (active vs. control). The quantitative indicators will calculate the mean and standard deviation. The counting data are statistically described by frequency (composition ratio). Differences between categorical variables were compared using a chi-square test or Fisher’s exact test with Yates’ correction if any cell number was less than five or close to zero. Differences between the nonnormally distributed continuous variables were compared using the nonparametric Mann–Whitney U test, while the normally distributed continuous variables were compared using an independent two-sample t-test. Differences between baseline and 20 weeks for continuous variables were compared using the paired t-test. For comparisons, unless otherwise specified, all statistical assessments were two-sided, and a P value of 0.05 was considered significant. Statistical analyses were performed with the SPSS software for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
During the study period, 100 patients in the active group and 50 patients in the control group underwent the study. In the active group, 12 patients were lost to follow-up and 7 were excluded due to adverse events or other reasons; in the control group, 8 were lost to follow-up and 2 were excluded due to adverse events or other reasons. One hundred twenty-one patients finished the 20-week treatment. One hundred twelve of these patients (76 in the active group and 36 in the control group) completed the QOL, mood, and cognition assessment at 20 weeks and were finally included in the final analysis (Fig. 1).
Patient demographics are shown in Table 1. There were no significant differences in age, gender, nationality, height, weight, and BMI between active and control groups. For medical history and baseline assessment, no significant differences were shown in years of epilepsy, seizure frequency, quality of life (QOL), mood and cognition assessment (QOLIE-31, HAMA, HAMD, MINI, MoCA scores), vital signs, and neurological examinations between the two groups (Table 1). Baseline medications of patients in the two groups are also shown in Table 1. The ASM regimen was maintained throughout the study.
Efficacy
Comparison of the responder rate at 4, 12, and 20 weeks between the active and control groups is shown in Fig. 4, The responder rate was significantly higher in active group (44.74%) than in control group (16.67%) at 20 weeks (P < 0.05). There were no significant differences in the responder rate at 4 weeks (22.67% vs 25.00%) and 12 weeks (28.95% vs 30.56%) between the two groups. Figure 5 shows the comparison of seizure frequency between active and control groups at each time point. No significant differences were shown between the two groups (active vs control) at baseline (4.01 ± 4.43 vs 7.29 ± 11.40, P = 0.104), 4 weeks (3.70 ± 4.79 vs 7.16 ± 13.33, P = 0.139), 12 weeks (3.29 ± 4.56 vs 7.07 ± 13.72, P = 0.115), and 20 weeks (3.08 ± 4.62 vs 6.05 ± 11.07, P = 0.130) all time points.
Baseline seizure frequency (mean ± SD) was 4.01 ± 4.43 in the active group and 7.29 ± 11.40 in the control group, respectively. At the end of treatment (20 weeks), mean seizure frequency (n/month) declined by 0.93 (P = 0.207) in the active group and 1.24 (P = 0.641) in the control group compared to the baseline. The percentage of seizure reduction in the active group (30.75% ± 54.32%) was significantly higher than that in the control group (15.66% ± 44.92%) at 20 weeks (P < 0.05) (Fig. 6A and Table 2). The proportions of different levels of seizure frequency reduction in active and control group at 4, 12, and 20 weeks are shown in Fig. 6B. There was significant difference between the two groups at 20 weeks (P < 0.05). No significant differences were shown at 4 weeks (P = 0.412) and 12 weeks (P = 0.401) between the two groups.
A Mean relative reduction in seizure frequency at each time point as compared to baseline seizure frequency: a significant relative reduction in seizure frequency was shown in the active group compared to the control group. B The level of seizure frequency reduction in active and control group at 4, 12, and 20 weeks (A): there was significant difference between the two groups at 20 weeks (P < 0.05)
Figure 7 shows the comparison of QOLIE-31, HAMA, HAMD, MINI, and MoCA scores (mean ± SD) at baseline and 20 weeks in active and control groups. The scores at baseline and 20 weeks as well as changes in scores are displayed in Table 4. The differences of score changes in QOLIE-31, HAMA, HAMD, MINI, and MoCA between baseline and 20 weeks were not significant (Table 3).
Safety
Adverse events (AE) during the treatment period were recorded to evaluate the safety of ta-VNS. During the treatment period, 10 patients (11.36%) from the active group experienced 13 AEs and 9 patients (19.57%) from the control group experienced 15 AEs. None of them were rated as severe AEs. No significant differences were shown between the two groups (P = 0.204) (Table 4). The most frequent AEs included headache, insomnia, and flu-like symptoms (mild fever, fatigue, runny nose, sore throat). Specifically, 2 patients from the active group experienced 2 device-related AEs such as ear erythema while 1 patient from the control group experienced 1 device-related AE. No severe device-related AEs were reported in either group. No significant differences were shown between the two groups (Table 2). Only 1 patient in the active group reported sinus bradycardia suggested by ECG at 12 weeks without obvious discomfort, which might be considered due to parasympathetic activation by stimulation procedure. This patient’s heart rate returned to normal range at 20 weeks, the final visit of the study. No relevant changes in vital signs were noted at any visit during the study. No abnormal findings with respect to vital signs were reported as adverse events either.
Discussion
The present study was a randomized, double-blind controlled trial of ta-VNS for the treatment of epilepsy, which examined the effect and safety for 20 weeks of stimulation. The result of the study suggested significant higher responder rate along with no significant increase in adverse events in patients receiving ta-VNS treatment.
In the present study, the responder rate was 44.74% in the active stimulation group at 20 weeks of treatment, which was significantly higher than that in the control group (16.67%). Seizure frequency decreased significantly by 30.75% on average in the active stimulation group who completed the intended 20 weeks of ta-VNS treatment, which was also significantly higher than that in the control group. In the early double-blinded, controlled i-VNS studies, total seizure frequency reduction could reach to 24.5–28.0%, which was significantly higher than the actively controlled group (6.1–15.0%) [6, 19]. As a noninvasive VNS treatment, the efficacy of ta-VNS has been investigated in previous work. In an animal study, ta-VNS was found to be equally effective as i-VNS in pentylenetetrazole-induced seizure model [20]. Furtherly, a number of clinical trials of ta-VNS for treatment of epilepsy have been published previously [13, 15, 16, 21, 22]. Rong et al. found that ta-VNS could effectively reduce seizure frequency and severity in patients with drug-resistant epilepsy [22]. Aihua et al. found that the monthly seizure frequency was lower in the treatment group than in the control group after 12-month treatment, and this reduction in seizure frequency was associated with baseline seizure frequency and duration of epilepsy [16]. Bauer et al. suggested no significant differences between high-frequency (25 Hz) and low-frequency (1 Hz) stimulation groups in responder rate (seizure reduction ≥ 50%, 27.0% vs 25.6%). However, they found a significant reduction in seizure frequency in patients of the 25 Hz group who completed the 20-week treatment period in contrast to 1 Hz group [15]. In the present study, we used the same stimulation parameters and study period as the high-frequency group in Bauer’s study for the active stimulation group and achieved a responder rate (seizure reduction ≥ 50%) of 44.74%. This discrepancy in responder rate might be due to the differences in study population, baseline seizure frequency, and duration of epilepsy between the two studies. Based on the above results, the anti-seizure effect of high-frequency ta-VNS treatment could be furtherly proven in this study [22]. Further studies should be performed focusing on comparison between i-VNS and ta-VNS and between different stimulation paradigms of ta-VNS, which should include longer follow-up period.
According to the data from 3 treatment visits, we found that the efficacy of ta-VNS appeared to increase with treatment time and seizure frequency reduction finally reached to 30.75% in the active group at the end of 20-week treatment, while the control group did not show such typical trend. This increase in anti-seizure effect over time was comparable to previous studies of ta-VNS [12, 15, 16]. In a previous study with a longer treatment period, seizure frequency was decreased in the treatment group by about 40% after 12-month treatment while increased by 0.85% in the control group [16]. This might suggest patients could get more anti-seizure benefits from long-period ta-VNS treatment. Nevertheless, whether long-period ta-VNS is associated with more adverse effects and whether this treatment could be well tolerated still needed to be investigated in future studies. Furthermore, the 20-week seizure frequency reduction in our study and 12-month seizure frequency reduction in Aihua’s study were both lower than that in Bauer’s study at 20 weeks [15, 16]. However, our study and Aihua’s study used patients with relatively lower baseline seizure frequency which was previously found to be associated with efficacy. Thus, this might account for the lower effect in seizure reduction.
In previous studies, patients receiving ta-VNS treatment and experiencing seizure frequency reduction showed significantly increased QOL scores compared with baseline [15, 16, 22]. However, whether the improvement of QOL is correlated with seizure reduction is controversial. In some studies, it was found that the increase in QOL scores had no correlation with reduction in seizure frequency. Aihua et al. showed that a number of patients under ta-VNS experienced improvement in QOL despite no significant seizure reduction [16]. These suggested the beneficial effect of ta-VNS on QOL might be independent of its antiepileptic effect. In addition, several studies found that the improvement in QOL of epileptic patients was mainly correlated with seizure freedom rather than just a reduction in seizure frequency [23]. However, in our study, patients in the active stimulation group did not show significant improvement in QOL despite significant seizure reduction. This might be due to relatively low seizure frequency and high QOL scores at baseline. Therefore, the effect of ta-VNS on QOL in treating epilepsy should be furtherly investigated in the future.
The beneficial effect of VNS on mood disorders such as anxiety and depression has been demonstrated in previous animal studies [24, 25] and clinical trials [26,27,28]. VNS mainly stimulates the afferent fibers which are anatomically and functionally connected with the target regions such as limbic system to achieve improvement in mood. In its mode of action, VNS could modulate the concentrations of neurotransmitters such as serotonin, norepinephrine, glutamate and GABA, and their metabolites while leading to functional changes in CNS, which makes VNS produce similar effects as most anti-anxiety and anti-depression agents [29]. Specifically, several studies showed that ta-VNS could improve anxiety/depression conditions in addition to its antiepileptic effect [15, 16, 26], and ta-VNS has been approved in Europe for the treatment of epilepsy and depression [30]. However, this effect mostly took a relatively long period up to 12–24 months to become significant. In our study, no significant improvement was shown in neither anxiety nor depression scores in patients receiving ta-VNS. One possible reason was the limited sample size and follow-up period and the other was the relatively mild level of mood disorders (low HAMA and HAMD scores).
The evidence of beneficial effects of VNS on cognitive functions is relatively limited and mainly from animal studies [31,32,33]. Although a small number of clinical studies reported improvement in cognitive functions by VNS [34, 35], the cognition improving effect of ta-VNS as an antiepileptic treatment still need to be investigated in future studies. In addition, the tool for cognitive function evaluation in this study was MoCA, which was usually used for dementia screening and might not be completely suitable for the cognition assessment of epileptic patients. This could be a possible limitation of this study.
Generally, ta-VNS treatment was well tolerated in this study. The most frequent AEs included headache, insomnia, flu-like symptoms, and ear erythema. The quantity and quality of AEs were comparable to what was found in previous studies [12, 15, 16]. Only 1 patient in the active stimulation group reported asymptomatic mild sinus bradycardia by ECG. This cardiac arrhythmias during ta-VNS treatment might be due to the parasympathetic innervation of the heart by the stimulation of vagus nerve and appeared to be transient in this study, which was not considered adverse event affecting the outcomes. However, cardiac abnormality related to ta-VNS should be paid more attention in the future studies. In addition, Ta-VNS appeared to cause less AEs such as nausea, pharyngitis, and hoarseness, which were regarded as common AEs of i-VNS related to invasive implantation procedure [19, 36]. The advantages of ta-VNS as a noninvasive treatment over i-VNS are needed to be furtherly investigated.
There are some limitations of our study. Firstly, the sample size in our study was not large enough to perform subgroup analysis stratified by etiology, ASM type, or level of seizure frequency, all of which might affect the efficacy of ta-VNS. The relatively high dropout rate (25.3%) might also challenge the statistical analysis, mainly resulting from loss to follow-up, especially for patients who lived in remote areas and had difficulties in finishing the whole study. Thus, studies with larger sample size and necessary subgroup analysis should be furtherly performed. Secondly, for efficacy analysis, our study mainly focused on seizure frequency, and there was no comparison of seizure severity between groups. More comprehensive assessment of epilepsy condition is needed in future studies. Thirdly, analysis of ictal and interictal EEGs was lacking in our study due to incomplete EEG data from the follow-up. In addition, another limitation of this study, which is similar to most clinical studies of epilepsy, is the possible inaccuracy of seizure frequency caused by the use of seizure diaries. Although such inaccuracy is relatively constant intraindividually throughout the study period and bring little influence on the difference analysis, future studies should use more accurate methods such as video EEG in quantifying seizure events.
Conclusions
In view of the significant higher responder rate along with no significant increase in adverse events, the present study showed that ta-VNS is an effective and safe therapy for epilepsy. Furthermore, the benefit in QOL, mood, and cognitive state of ta-VNS needs further validation in the future study although no significant improvement was shown in this study. We believe that ta-VNS may be a promising noninvasive treatment options for patients with epilepsy, especially for those resistant to ASMs.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
References
Dua T, de Boer HM, Prilipko LL, Saxena S. Epilepsy care in the world: results of an ILAE/IBE/WHO Global Campaign Against Epilepsy survey. Epilepsia. 2006;47(7):1225–31.
Saxena S, Li S. Defeating epilepsy: a global public health commitment. Epilepsia Open. 2017;2(2):153–5.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9.
Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021;20(12):1038–47.
Toffa DH, Touma L, El Meskine T, Bouthillier A, Nguyen DK. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review. Seizure. 2020;83:104–23.
Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, First International Vagus Nerve Stimulation Study Group, et al. Vagus nerve stimulation for treatment of partial seizures: 1. a controlled study of effect on seizures. Epilepsia. 1994;35(3):616–26.
Gonzalez HFJ, Yengo-Kahn A, Englot DJ. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg Clin N Am. 2019;30(2):219–30.
Schulze-Bonhage A. Long-term outcome in neurostimulation of epilepsy. Epilepsy Behav. 2019;91:25–9.
Kim JS, Kim DY, Jo HJ, Hwang YH, Song JY, Yang KI, et al. Effect of long-term treatment with vagus nerve stimulation on mood and quality of life in korean patients with drug-resistant epilepsy. J Clin Neurol. 2021;17(3):385–92.
Mao H, Chen Y, Ge Q, Ye L, Cheng H. Short- and long-term response of vagus nerve stimulation therapy in drug-resistant epilepsy: a systematic review and meta-analysis. Neuromodulation. 2021.
Lampros M, Vlachos N, Zigouris A, Voulgaris S, Alexiou GA. Transcutaneous vagus nerve stimulation (t-VNS) and epilepsy: a systematic review of the literature. Seizure. 2021;91:40–8.
Rong P, Liu A, Zhang J, Wang Y, Yang A, Li L, et al. An alternative therapy for drug-resistant epilepsy: transcutaneous auricular vagus nerve stimulation. Chin Med J (Engl). 2014;127(2):300–4.
He W, Jing X, Wang X, Rong P, Li L, Shi H, et al. Transcutaneous auricular vagus nerve stimulation as a complementary therapy for pediatric epilepsy: a pilot trial. Epilepsy Behav. 2013;28(3):343–6.
Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 2012;53(7):e115–8.
Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimul. 2016;9(3):356–63.
Aihua L, Lu S, Liping L, Xiuru W, Hua L, Yuping W. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 2014;39:105–10.
Farmer AD, Strzelczyk A, Finisguerra A, Gourine AV, Gharabaghi A, Hasan A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2020;14:568051.
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51(1):48–55.
Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16(2):101–2.
He W, Wang XY, Zhou L, Li ZM, Jing XH, Lv ZL, et al. Transcutaneous auricular vagus nerve stimulation for pediatric epilepsy: study protocol for a randomized controlled trial. Trials. 2015;16:371.
Rong P, Liu A, Zhang J, Wang Y, He W, Yang A, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: a randomized controlled trial. Clin Sci (Lond). 2014.
Birbeck GL, Hays RD, Cui X, Vickrey BG. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43(5):535–8.
Noble LJ, Chuah A, Callahan KK, Souza RR, McIntyre CK. Peripheral effects of vagus nerve stimulation on anxiety and extinction of conditioned fear in rats. Learn Mem. 2019;26(7):245–51.
Iannucci J, Nizamutdinov D, Shapiro LA. Neurogenesis and chronic neurobehavioral outcomes are partially improved by vagus nerve stimulation in a mouse model of Gulf War illness. Neurotoxicology. 2022;90:205–15.
Genheimer H, Andreatta M, Asan E, Pauli P. Reinstatement of contextual conditioned anxiety in virtual reality and the effects of transcutaneous vagus nerve stimulation in humans. Sci Rep. 2017;7(1):17886.
Zhang B, Wang W, Wang S, Li S, Liu M, Wang L, et al. Clinical study on electronic medical neuroelectric stimulation based on the Internet of Things to treat epilepsy patients with anxiety and depression. J Healthc Eng. 2021;2021:6667309.
Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266–73.
Muller HHO, Moeller S, Lucke C, Lam AP, Braun N, Philipsen A. Vagus nerve stimulation (VNS) and other augmentation strategies for therapy-resistant depression (TRD): review of the evidence and clinical advice for Use. Front Neurosci. 2018;12:239.
Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part II. Headache. 2016;56(2):259–66.
Venkatasamy L, Nizamutdinov D, Jenkins J, Shapiro LA. Vagus nerve stimulation ameliorates cognitive impairment and increased hippocampal astrocytes in a mouse model of Gulf War illness. Neurosci Insights. 2021;16:26331055211018456.
Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. 2019;12(1):19–29.
Vazquez-Oliver A, Brambilla-Pisoni C, Domingo-Gainza M, Maldonado R, Ivorra A, Ozaita A. Auricular transcutaneous vagus nerve stimulation improves memory persistence in naive mice and in an intellectual disability mouse model. Brain Stimul. 2020;13(2):494–8.
Knorr C, Greuter L, Constantini S, Fried I, Kremer U, Datta AN, et al. Subgroup analysis of seizure and cognitive outcome after vagal nerve stimulator implantation in children. Childs Nerv Syst. 2021;37(1):243–52.
Sun L, Perakyla J, Holm K, Haapasalo J, Lehtimaki K, Ogawa KH, et al. Vagus nerve stimulation improves working memory performance. J Clin Exp Neuropsychol. 2017;39(10):954–64.
Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260–8.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported by the National Key R&D Program of China 2022YFC2503800, Beijing Postdoctoral Research Foundation (ZZ2019-09 and ZZ2020-06), China Postdoctoral Science Foundation (no. 2019M660719), and Beijing Nova Program (Z211100002121047). The Capital Health Research and Development of Special (2020-1-2013), Beijing Natural Science Foundation (7232045 and Z200024).
Author information
Authors and Affiliations
Contributions
HY and WS participated and was responsible for literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical approval of the final report, and funding, considered first author. WS, JF, XW, YS, YL, and QW had full access to the data and take responsibility for data collection, the integrity of the data, and the accuracy of analysis. QW was responsible for the final approval of the article.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Shi, W., Fan, J. et al. Transcutaneous Auricular Vagus Nerve Stimulation (ta-VNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial. Neurotherapeutics 20, 870–880 (2023). https://doi.org/10.1007/s13311-023-01353-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01353-9