Abstract
Outcomes of laparoscopic liver resection (LLR) versus open LR (OLR) for intrahepatic cholangiocarcinoma (ICCA) are heterogeneous. We aimed to compare LLR and OLR for ICCA based on propensity-score-matched (PSM) studies. Two reviewers independently searched the online databases (PubMed, Embase, and Cochrane Library) for PSM studies that compared LLR and OLR for ICCA. The Ottawa–Newcastle Quality Assessment Scale with a cutoff of ≥ 7 was used to define higher-quality literature. Only ‘high-quality’ PSM analyses of the English language that met all our inclusion criteria were considered. A total of ten PSM trials were included in the analyses. Compared with OLR, although the lymph node dissection (LND) (RR = 0.67) and major hepatectomy rates were lower in the LLR group (RR = 0.87), higher R0 resections (RR = 1.05) and lower major complications (Clavien–Dindo grade ≥ III) (RR = 0.72) were also observed in the LLR group. In addition, patients in the LLR group showed less estimated blood loss (MD = − 185.52 ml) and shorter hospital stays as well (MD = − 2.75 days). Further analysis found the overall survival (OS) (HR = 0.91), disease-free survival (DFS) (HR = 0.95), and recurrence-free survival (HR = 0.80) for patients with ICCA after LLR were all comparable to those of OLR. LLR for selected ICCA patients may be technically safe and feasible, providing short-term benefits and achieving oncological efficacy without compromising the long-term survival of the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICCA) and hepatocellular carcinoma (HCC) are the most seen primary liver cancers (PLC). Studies reported most PLCs were HCC (> 80%), and ICCA only accounted for 10–15% [1]. Compared with HCC, ICCA is rarer, and there is a lack of large population-based or randomized-controlled studies (RCT) of ICCA treatment. Reviewing existing literature for evidence of ICCA treatment is required [2,3,4,5].
There are significant differences between HCC and ICCA [6] when regarding the patient's basic demographic characteristics and tumor biological features. Patients with ICCA usually had a worse prognosis, with 5-year overall survival (5-OS) being 20–40% versus that of 50–70% for patients with HCC. Studies have also found that ICCA patients tend to present a more advanced tumor burden compared to HCC, including a higher rate of lymph node involvement (27.6% vs. 7.7%) and larger tumor size (proportion of tumors ≥ 5 cm (63.7% vs. 46.3%)) [1]. Therefore, although laparoscopic liver resection (LLR) is gaining momentum for HCC [1, 7, 8], evidence of LLR application for ICCA has not been quantified in the aggregate [9,10,11,12,13].
Due to inferior tumor biological behaviors, the difficulty of hilar lymph node dissection (LND), and hepato-biliary reconstruction, LLR application for ICCA is in the initial phase and mainly reserved in high-volume experienced medical centers. Most existing studies including meta-analyses (MAs) had reported LLR for selected ICCA patients was technically safe and feasible, with comparable oncological outcomes to the open LR (OLR) [10, 14, 15]. Contradictory results were still reported from several studies [16].The safety, feasibility, and oncological efficiency of LLR for ICCA are related to ICCA tumor stages and the surgeons’ experience. Therefore, significant heterogeneity is presented in published studies. Moreover, postoperative complications and survival outcomes after either LLR or OLR may vary with the availability of efficacious antitumor therapies and improved perioperative management over time. Thus, published systematic reviews need to be updated with high-quality propensity-score-matched (PSM) studies.
Based on the reasons above, we aimed to compare both perioperative-related and long-term survival outcomes of LLR and OLR in patients with ICCA through a meta-analytic approach. Only recently published PSM studies of high quality were included in analyses.
Materials and methods
Literature search
The protocol for this study had been registered at PROSPERO (CRD42022348032). Our paper followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [17].
Two reviewers (Author 1 and Author 2) systematically searched online databases including PubMed, Cochrane Library, and Embase from data inception to May 12, 2023, to find studies that might be suitable for our meta-analyses. The search strategy was based on the PICOS principle, combining MESH and their associated/related terms. The search items were presented as follows: ‘Intrahepatic cholangiocarcinoma [MeSH Terms]’ and ‘open liver resection [MeSH Terms]’ and ‘laparoscopic liver resection [MeSH Terms]’ and ‘propensity score-matched analyses [MeSH Terms]’ or ‘open liver surgery’ or ‘open hepatectomy’ or ‘minimally invasive liver resection’ or ‘minimally invasive hepatectomy’ or ‘laparoscopic hepatectomy’ or ‘PSM analyses’ or ‘matched studies’ or ‘ICCA’ or ‘Intrahepatic cholangiocarcinomas’. More detailed search terms are presented in Supplementary Table S3.
The entire texts of possibly qualifying papers were reviewed by the same 2 reviewers (Author 1 and Author 2) separately. A third reviewer (Corresponding Author) was consulted to settle any disagreements met. To avoid literature that might be ignored during our search, we reviewed the citations of retrieved eligible articles as well as the conference proceedings manually. Besides, to avoid missing studies that were published after our first search, we performed re-research before the submission on June 15, 2023.
Inclusion criteria
Studies that were eligible for the meta-analyses should meet the following criteria:
-
i.
PSM studies of the English language.
-
ii.
All including patients were pathologically diagnosed with ICCA after postoperative pathological diagnosis.
-
iii.
Use of LLR or OLR for curative-intent resection of ICCA.
-
iv.
Comparing LLR versus OLR for ICCA with sufficient data including perioperative results, and long-term oncological outcomes.
-
v.
Full text available.
Papers were excluded if they were met any of the following:
-
a.
Non-English languages.
-
b.
Non-comparative analyses, animal, or laboratory studies.
-
c.
Abstracts only, meetings, books, or Letters to the Editors.
-
d.
Studies that lack adequate clinical data.
-
e.
Studies included patients with combined pathologies besides ICCA such as ICCA combined with hepatocellular carcinoma or metastatic liver diseases were excluded. Studies reported patients with perihilar, distal cholangiocarcinoma, and gallbladder cancer were also not considered.
-
f.
Studies of low quality were also excluded from the analyses.
Quality assessments
The quality of the included studies was assessed independently at both the individual study level and outcome level by two reviewers. The Newcastle–Ottawa Scale (NOS) [18] was used to evaluate the risk of bias of each included PSM study The NOS assessment criteria give a maximum of 9 points for risk of bias in three areas: Selection of the cohort, Comparability of exposed and nonexposed participants, and Assessment of results. Literature with a score of ≥ 7 was considered to have a low risk of bias and then was classified as high quality. (Supplementary Table S2).
Data extraction
Data extraction was conducted independently by 2 reviewers (Author 1 and Author 2), with any conflicts referred to a third reviewer (Corresponding Author) for clarification.
We gathered data from the included PSM studies. Study characteristics including but not limited to author/year of publication, research duration, study type (multicenter/single center and retrospective/prospective), and study population were extracted and are presented in Table 1. The patients’ basic demographic features and tumor characteristics in each group (LLR and OLR) such as patients' median age, gender, presence of liver cirrhosis, tumors histology, tumor number, and median diameter can be found in Supplementary Table S1.
Definitions
To help comprehensive and accurate measurement of complications that occurred after LLR and OLR, only studies that reported complications graded according to the Clavien–Dindo classification were considered for analyses. Major complications were defined as Clavien–Dindo grade III/IV in the included studies. Textbook Outcome (TO) [12] was a new composite-outcome index. The included 2 studies both considered patients with negative margins (R0), without transfusion, no severe surgical complications, prolonged hospital stay, readmissions, and no postoperative mortality as to have a TO.
Statistical analyses
The primary objective of our meta-analysis was to assess the safety, feasibility, and long-term outcomes (OS, DFS, and RFS) for resected ICCA patients after LLR and OLR. The Risk ratios (RRs) were used to assess binary variables and mean differences (MDs) to assess continuous variables. The hazard ratio (HR) for survival outcomes was calculated using the statistical method of Tierney et al. If the pooled HR and its 95%CI (confidence interval) overlapped 1, LLR had statistically comparable survival effects to OLR.
Heterogeneity between studies was assessed using Cochran’s Q test and Higgins I2 statistic. I2 values of 25%, 50%, and 75% represented low, moderate, and high levels of heterogeneity, respectively. An I2 greater than 50% (I2 > 50%) was defined as the criterion for significant heterogeneity. Random-effect models were used if significant heterogeneity emerged after the analyses. Only if the analyses showed low and/or moderate heterogeneity (I2 ≤ 50%) were fixed-effects models considered. Sensitivity analysis using the leave-one-out method was performed to assess the robustness of the results for study results with significant heterogeneity.
Potential publication bias was statistically assessed using Egger’s linear regression. A P value of greater than 0.05 for Egger’s test indicated the absence of significant statistical publication bias. To determine the effect of individual studies on the pooled estimates, sensitivity analyses were performed by serially excluding each study. Statistical significance was defined as two-tailed P values less than 0.05.
All statistical analyses were performed using RevMan software 5.3 (The Cochrane Collaboration, Oxford, UK) and R software version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria), and the Meta package was used for data analysis.
Results
Study characteristics
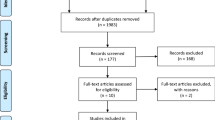
After comprehensive database searching and removing duplicates, 186 studies were originally identified. After a screening of titles and abstracts, 164 were removed for reasons of non-English language, single-arm analyses, case reports, reviews, letters, commentary, and conferences. Twelve studies were further evaluated for full text. Ten studies that met all inclusion criteria were finally included in the meta-analysis [16, 19,20,21,22,23,24,25,26,27]. The flow diagram of the literature inclusion is presented in Fig. 1.
All the included studies were retrospective. According to NOS, included literature was of high quality, with 3 studies obtaining a score of 9, and 7 having a score of 8. We included 2 studies by Ratti et al. [19, 20] for the patient population in these studies were from different regions (Milan and Two European referral centers), and the research duration was also different. We summarized the baseline characteristics of the included studies in Table 1. The main outcomes of the meta-analyses are presented in Table 2.
Although all included analyses were after the PSM method, given the differences in sample inclusion criteria among literature, we still compared whether important tumor characteristics differed significantly between the two groups (LLR and OLR). These indicators have been considered key factors that may influence the prognosis for ICCA patients in the previously published literature. There were no statistically significant differences in the basic demographics and tumor characteristics between the laparoscopic and open arms. Differences were noted in terms of patients in the LLR group having smaller tumor size versus OLR with SMD = − 0.13 (95% CI − 0.23 to − 0.03; P = 0.02; I2 = 0%). Both positive LN status and multiple tumor numbers (≥ 2) in the LLR group were comparable to OLR, with RR = 0.68 (95% CI 0.44–1.06, P = 0.09; I2 = 70%) and RR = 0.95; (95%CI: 0.76–1.20; P = 0.68; I2 = 0%), respectively. Significant heterogeneity was seen in the comparison of LN status in LLR versus OLR (I2 = 70%).
Perioperative outcomes
With a pooled RR = 0.87 (95%CI 0.78–0.97, P = 0.01; I2 = 19%), we found that the incidence of major hepatectomy was lower in LLR than in OLR (Fig. 2a). Regarding LN dissection (LND) rates, we also found that patients after LLR group had lower LND rates than those in the OLR group (RR = 0.67; 95% CI 0.49–0.91; P = 0.01). However, there was a significant degree of heterogeneity among the included studies with I2 = 91% (Fig. 2c). Although the major hepatectomy and LND rates were lower in the LLR group, we found patients in the LLR group achieved a higher incidence of R0 resection than those in the OLR group (RR = 1.05, 95%CI 1.01–1.09, P = 0.008; I2 = 29%) (Fig. 2b).
We also determined the postoperative outcomes for patients with ICCA after LLR and OLR. Our analyses showed that compared with the OLR group, the major complications (Clavien–Dindo grade ≥ III) rate was lower for ICCA patients after LLR with RR = 0.72 (95%CI 0.56–0.94, P = 0.01; I2 = 45%) (Fig. 3a). The 90-day mortality rate was comparable between the two arms with a pooled RR = 0.69 (95%CI 0.46–1.03, P = 0.07; I2 = 1%) (Fig. 3b). Textbook outcome (TO), a novel composite measure for the surgical care quality, which represents the ideal hospitalization for patients with ICCA undergoing curative-intent resection. To compare the difference in TO between LLR and OLR, we included 2 studies with the same definitions of TO. In the end, we found that the patients in the LLR group achieved a higher TO than the patients after OLR (RR = 1.42; 95%CI: 1.05–1.92, P = 0.02). No heterogeneity was found in the analyses with I2 = 0% (Fig. 3c).
To assess the value of using LLR for ICCA more comprehensively, we have summarized other perioperative outcomes as follows: LLR resulted in lower blood loss (MD = − 185.82 ml), shorter hospital stays (MD = − 2.75 days), less lymphatic fistula incidence (RR = 0.29), and lower perioperative blood transfusion rate (RR = 0.45) than the OLR group. In addition, there was no significant heterogeneity in most of the outcomes. Nevertheless, the number of LNs harvested (MD = 0.24), the duration of surgery (MD = 10.53 min), the incidence of liver failure (RR = 0.64), and the bile leakage (RR = 0.55) rate for the patients in the LLR group were comparable to patients after OLR. The pooled results are shown in Supplementary Appendix: Figure S1, Figure S2. Finally, the conversion rate of laparoscopic procedures was reported in 7 studies and ranged between 0% and 18.0%, which is slightly lower than the results in other published literature (7.4–20%).
Postoperative long-term outcomes
Regarding the recurrence-free survival (RFS) in ICCA patients after surgery, no difference was found between LLR and OLR. Patients in the LLR group had comparable RFS compared to those with OLR (HR = 0.80, 95%CI 0.63–1.02, P = 0.07; I2 = 0%. Figure 4a). No significant difference was also resulted between LLR and OLR regarding either overall survival (OS) (HR = 0.91; 95% CI 0.81–1.03, P = 0.14; I2 = 0%; Fig. 4b) or disease-free survival (DFS) (HR = 0.95, 95% CI 0.80–1.14; P = 0.60; I2 = 38%; Fig. 4c), and there was no significant heterogeneity among the studies (I2 = 0% and 38%, respectively).
Sensitivity analyses and publication bias
Results with significant heterogeneity were pooled with the random-effect model and performed with sensitivity analyses. In our study, substantial heterogeneity was observed in the analyses of the hospital stay, surgery duration, and LND rate. After performing the sensitivity analysis using the leave-one-out method, we found no changes in the risk estimate or the level of significance in terms of these outcomes. The significant heterogeneity among the included studies may be related to the surgeon's varied experiences, different hospital volumes, and LND indications. For the assessment of publication bias, we performed Egger's test and found no significant bias in terms of length of hospital stay (P = 0.2168), surgery duration (P = 0.4558), and LND rate (P = 0.0652) (Supplementary Figure S2a–c). However, due to the limited study samples (N = 3), the publication bias for retrieved lymph nodes number objectively exists.
Discussions
For patients with ICCA, radical surgery resection for a negative margin (R0) is the only curative treatment [28, 29]. Due to the technical difficulty and uncertainty in long-term efficacy, most of the ICCA surgeries are performed under an open surgery method. LLR for ICCA is just starting, and uncertainty that exists over the subject of ICCA can be routinely solved through an LLR procedure. Only limited high-volume centers had demonstrated the feasibility and safety of LLR for ICCA. LLR technique for ICCA remained challenging at most medical centers, particularly when dealing with large (> 5 cm), multiple (> 2), or advanced ICCA tumors [11, 13, 19, 30, 31].
Published studies included different stages of ICCA in LLR and OLR; thus, the baseline characteristics of the patients were not totally matched [20, 21]. After analyzing, we found in most of the current literature, ICCA patients treated with the laparoscopy method usually had tumors of early stages and smaller sizes; thus, there is less need for large-scale hepatectomy or LND. Owing to the earlier staging of ICCA within the LLR group, previous research has not definitively established the advantages of LLR for ICCA, when compared with the OLR arm [32, 33]. Therefore, in our study, we only included PSM studies for the comparison of LLR versus OLR.
Previous studies had reported some factors including R0 resections, large tumor size, and positive LN status may influence the OS of ICCA patients. This interaction on the hand demonstrated that LLR should be performed in selected ICCA with the feasibility of adequate tumor resection and LND [20, 31, 33]. Our study found that LLR for some selected ICCA cases resulted in similar oncological and long-term survival outcomes to those of OLR, with the advantages of less blood loss, major complication rate, and shorter hospital stays. Prior studies have also shown that LLR could confer better short-term perioperative outcomes to OLR with comparable long-term survival prognosis [14, 15]. Due to smaller incisions, both wound complication rate and postoperative pain were significantly decreased in the LLR group which also help to improve the patient’s recovery. Regarding similar oncological outcomes including R0 resection and major hepatectomy rate to OLR, LLR for some selected ICCA seemed to be the optimal method.
However, LLR was associated with inferior LND in most of the published studies [14, 15] which made the effectiveness of laparoscopic LND remained controversial [32]. Martin et al. showed the laparoscopic LND rate was significantly lower than that of open surgery (39% vs. 61%, P < 0.01); while other scholars believed that the magnifying effect of laparoscopy was helpful to identify LN for a comprehensive surgery resection. Ratti et al. showed that laparoscopic LND could result in a similar number of LN to open surgery with lower complications incidence related to LND. According to the guidelines, LND was acknowledged as a standard treatment for ICCA; those conflicting published results suggested that LLR for ICCA currently may not be totally optimal [16, 19, 34].
A meta-analysis conducted by Zhou et al. suggested that LND had a limited impact on the ICCA patient's long-term prognosis, besides a higher incidence of postoperative complications was seen in the LND group. In fact, the significance of LND in ICCA was to obtain more accurate pathological staging for guiding further adjuvant treatments. Therefore, experts from the American Hepatobiliary and Pancreatic Association recommend routine LND in the ICCA treatment and suggested that at least 6 lymph nodes should be obtained after surgical resection for tumor staging. LND was to obtain adequate numbers of LN, merely comparing the LND rate in OLR and LLR may not be as important as the compassion for the average number of retrieved LN in LLR and OLR. In our analyses, we resulted in lower LND rates for ICCA after LLR compared with OLR, while in further analyses, we demonstrated a comparable average number of retrieved LN in patients with LLR and OLR. Therefore, we may conclude that LLR for selected ICCA could be practiced at experienced centers with strict ongoing practice evaluation, and the oncological efficacy was not inferior to patients with OLR.
Although more and more medical centers have carried out LLR for ICCA, we should still pay attention to the surgical indications and strictly control the quality of the surgery. The objective of surgery is to ensure R0 resection and improve patients’ survival [35,36,37,38,39]. We still need large-scale multicenter studies to explore the safety, feasibility, surgery indications, contraindications, and long-term efficacy of LLR application in ICCA [12, 31, 32, 40,41,42,43,44]
Strengths and limitations
Our MAs provide an updated and thorough comparison of perioperative and long-term survival outcomes for ICCA patients following LLR and OLR. However, there are several limitations to our study. First, selection bias is inherent in our included retrospective studies, and publication bias is also unavoidable due to the limited number of studies in our MAs. Besides, although we only included studies of PSM methods, other features such as tumor stages or biology behaviors may not be strictly comparable in LLR and OLR, which may confound our results. Furthermore, given that only retrospective studies were available for our MAs, it may cause a higher level of heterogeneity. In addition, there are limited studies in our MAs, and the meta-regression for some baseline characteristics on patients’ survival is not actionable, reducing our MAs’ statistical power. Finally, data from Africa and Asia were scarce, which highlighted the need for more multicenter studies.
Conclusions
LLR for selected ICCA patients may be technically safe and feasible, providing short-term benefits and achieving oncological efficacy without compromising the long-term survival of the patients. Although an increasing number of medical centers have been performing LLR for ICCA, the procedure is still in the initial phase of exploration. More evidence is needed to validate the use of LLR for ICCA.
Data availability
All data generated or analyzed during this study are included in the published article.
Abbreviations
- ICCA:
-
Intrahepatic cholangiocarcinoma
- LLR:
-
Laparoscopic liver resection
- OLR:
-
Open liver resection
- PSM:
-
Propensity score matching
- MAs:
-
Meta-analyses
- LND:
-
Lymph node dissection
- PLC:
-
Primary liver cancer
- RCT:
-
Randomized-controlled studies
- TO:
-
Textbook outcome
References
Lee YT, Wang JJ, Luu M et al (2021) Comparison of clinical features and outcomes between intrahepatic cholangiocarcinoma and hepatocellular carcinoma in the United States. Hepatology 74(5):2622–2632
Mukund A, Srinivasan SV, Rana S et al (2022) Response evaluation of locoregional therapies in combined hepatocellular-cholangiocarcinoma and intrahepatic cholangiocarcinoma versus hepatocellular carcinoma: a propensity score matched study. Clin Radiol 77(2):121–129
Kendall T, Verheij J, Gaudio E et al (2019) Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int 39(Suppl 1):7–18
Bagante F, Spolverato G, Merath K et al (2019) Intrahepatic cholangiocarcinoma tumor burden: a classification and regression tree model to define prognostic groups after resection. Surgery 166(6):983–990
Mavros MN, Economopoulos KP, Alexiou VG et al (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149(6):565–574
Liu WR, Tian MX, Tao CY et al (2020) Adjuvant Transarterial chemoembolization does not influence recurrence-free or overall survival in patients with combined hepatocellular carcinoma and Cholangiocarcinoma after curative resection: a propensity score matching analysis. BMC Cancer 20(1):642
Birgin E, Kaslow SR, Hetjens S et al (2021) Minimally invasive versus open liver resection for stage I/II hepatocellular carcinoma. Cancers (Basel) 13(19):4800
Levi Sandri GB, Colasanti M, Aldrighetti L et al (2022) Is minimally invasive liver surgery a reasonable option in recurrent HCC? A snapshot from the I Go MILS registry. Updates Surg 74(1):87–96
Mejia JC, Pasko J (2020) Primary liver cancers: intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg Clin North Am 100(3):535–549
Patrone R, Izzo F, Palaia R et al (2021) Minimally invasive surgical treatment of intrahepatic cholangiocarcinoma: a systematic review. World J Gastrointest Oncol 13(12):2203–2215
Shiraiwa DK, Carvalho P, Maeda CT et al (2020) The role of minimally invasive hepatectomy for hilar and intrahepatic cholangiocarcinoma: a systematic review of the literature. J Surg Oncol 121(5):863–872
Görgec B, Benedetti Cacciaguerra A, Lanari J et al (2021) Assessment of textbook outcome in laparoscopic and open liver surgery. JAMA Surg 156(8):e212064
Machairas N, Lang H, Jayant K et al (2020) Intrahepatic cholangiocarcinoma: limitations for resectability, current surgical concepts and future perspectives. Eur J Surg Oncol 46(5):740–746
Regmi P, Hu HJ, Paudyal P et al (2021) Is laparoscopic liver resection safe for intrahepatic cholangiocarcinoma? A meta-analysis. Eur J Surg Oncol 47(5):979–989
Ziogas IA, Esagian SM, Giannis D et al (2021) Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma: an individual patient data survival meta-analysis. Am J Surg 222(4):731–738
Hobeika C, Cauchy F, Fuks D et al (2021) Laparoscopic versus open resection of intrahepatic cholangiocarcinoma: nationwide analysis. Br J Surg 108(4):419–426
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Lo CK, Mertz D, Loeb M (2014) Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 14:45
Ratti F, Casadei-Gardini A, Cipriani F et al (2021) Laparoscopic surgery for intrahepatic cholangiocarcinoma: a focus on oncological outcomes. J Clin Med 10(13):2828
Ratti F, Rawashdeh A, Cipriani F et al (2021) Intrahepatic cholangiocarcinoma as the new field of implementation of laparoscopic liver resection programs. A comparative propensity score-based analysis of open and laparoscopic liver resections. Surg Endosc 35(4):1851–1862
Brustia R, Laurent A, Goumard C et al (2022) Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: report of an international multicenter cohort study with propensity score matching. Surgery 171(5):1290–1302
Jinhuan Y, Yi W, Yuanwen Z et al (2021) Laparoscopic versus open surgery for early-stage intrahepatic cholangiocarcinoma after mastering the learning curve: a multicenter data-based matched study. Front Oncol 11:742544
Salehi O, Kazakova V, Vega EA et al (2022) Selection criteria for minimally invasive resection of intrahepatic cholangiocarcinoma-a word of caution: a propensity score-matched analysis using the national cancer database. Surg Endosc 36(7):5382–5391
Kang SH, Choi Y, Lee W et al (2020) Laparoscopic liver resection versus open liver resection for intrahepatic cholangiocarcinoma: 3-year outcomes of a cohort study with propensity score matching. Surg Oncol 33:63–69
Zhu Y, Song J, Xu X et al (2019) Safety and feasibility of laparoscopic liver resection for patients with large or multiple intrahepatic cholangiocarcinomas: a propensity score-based case-matched analysis from a single institute. Medicine (Baltimore) 98(49):e18307
Sahakyan MA, Aghayan DL, Edwin B (2023) Laparoscopic versus open liver resection. For intrahepatic cholangiocarcinoma: a multicenter propensity score-matched study. Scand J Gastroenterol 58(5):489–496 (Epub 2022 Nov 14)
Shen Z, Tao L, Cai J, Zheng J, Sheng Y, Yang Z, Gong L, Song C, Gao J, Ying H, Xu J, Liang X (2023) Safety and feasibility of laparoscopic liver resection for intrahepatic cholangiocarcinoma: a propensity score-matched study. World J Surg Oncol 21(1):126
Rizvi S, Khan SA, Hallemeier CL et al (2018) Cholangiocarcinoma—evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 15(2):95–111
Kelley RK, Bridgewater J, Gores GJ et al (2020) Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol 72(2):353–363
Mazzaferro V, Gorgen A, Roayaie S et al (2020) Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 72(2):364–377
Cillo U, Fondevila C, Donadon M et al (2019) Surgery for cholangiocarcinoma. Liver Int 39(Suppl 1):143–155
Wu J, Han J, Zhang Y et al (2020) Safety and feasibility of laparoscopic versus open liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma. Biosci Trends 14(5):376–383
Lee W, Park JH, Kim JY et al (2016) Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 30(11):4835–4840
Kim-Fuchs C, Candinas D, Lachenmayer A (2021) The role of conventional and stereotactic microwave ablation for intrahepatic cholangiocarcinoma. J Clin Med 10(13):2963
Wei F, Lu C, Cai L et al (2017) Can laparoscopic liver resection provide a favorable option for patients with large or multiple intrahepatic cholangiocarcinomas? Surg Endosc 31(9):3646–3655
Wang Y, Li J, Xia Y et al (2013) Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31(9):1188–1195
Kinoshita M, Kanazawa A, Takemura S et al (2020) Indications for laparoscopic liver resection of mass-forming intrahepatic cholangiocarcinoma. Asian J Endosc Surg 13(1):46–58
Panayotova GG, Guarrera JV, Lunsford KE (2020) Should we reevaluate liver transplantation as an alternative to resection for the treatment of intrahepatic cholangiocarcinoma? Liver Transpl 26(6):748–750
Ji GW, Jiao CY, Xu ZG et al (2022) Development and validation of a gradient boosting machine to predict prognosis after liver resection for intrahepatic cholangiocarcinoma. BMC Cancer 22(1):258
Farges O, Regimbeau JM, Fuks D et al (2013) Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 100(2):274–283
Molina V, Ferrer-Fábrega J, Sampson-Dávila J et al (2020) Intention-to-treat curative liver resection in patients with “very early” intrahepatic cholangiocarcinoma. Langenbecks Arch Surg 405(7):967–975
Sapisochin G, Ivanics T, Heimbach J (2022) Liver Transplantation for intrahepatic cholangiocarcinoma: ready for prime time? Hepatology 75(2):455–472
Moustafa M, Fasolo E, Bassi D et al (2020) The impact of liver resection on survival for locally advanced intrahepatic cholangiocarcinoma tumors: a propensity score analysis. Eur J Surg Oncol 46(4 Pt A):632–637
Tarchi P, Tabrizian P, Prigoff J et al (2018) Outcomes of resection for solitary ≤5 cm intrahepatic cholangiocarcinoma. Surgery 163(4):698–702
Funding
This study was funded by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21046); 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001); Natural Science Foundation of Sichuan Province (2022NSFSC0806); National Natural Science Foundation of China for Young Scientists Fund (82203650, 82203782), Sichuan Science and Technology Program (2021YJ0132, 2021YFS0100); The fellowship of China Postdoctoral Science Foundation (2021M692277); Sichuan University-Zigong School-local Cooperation project (2021CDZG-23); Science and Technology project of the Health planning committee of Sichuan (21PJ046); Sichuan University-Sui Lin School-local Cooperation project (2022CDSN-18); Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH127).
Role of the Funding Source: The funding Source has no role in the design and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Hu YF and Hu HJ contributed equally to the manuscript and were the first co-authors. Hu YF and Hu HJ contributed to data acquisition and drafted the manuscript. Jin YW and Ma WJ contributed to data acquisition. Li FY contributed to the study design and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13304_2023_1648_MOESM1_ESM.docx
Supplementary file1 (DOCX 22 KB). Supplementary Table S1. Patients’ basic demographics and tumor characteristics after PSM analyses. Supplementary Table S2. The Newcastle Ottawa Scale (NOS) of included studies. Supplementary Table S3. Detailed Search strategies.
13304_2023_1648_MOESM2_ESM.docx
Supplementary file2 (DOCX 12995 KB). Supplementary Fig S1a. Retrieved lymph nodes (LN) of LLR versus OLR. b. Liver failure after LLR versus OLR. c. Duration of hospital stay (days) of LLR versus OLR. d. Lymphatic fistula rates of LLR versus OLR. e. Biliary leakage rate of LLR versus OLR. f. Blood loss (ml) of LLR versus OLR. g. Perioperative blood transfusion rate of LLR versus OLR. h. Duration of surgery (min) of LLR versus OLR. Supplementary Fig S2a-c. Publication bias of length of hospital stay. a. Publication bias of length of hospital stay. b. Publication bias of length of surgery duration. c. Publication bias LND rate.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, YF., Hu, HJ., Ma, WJ. et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: a systematic review of propensity score-matched studies. Updates Surg 75, 2049–2061 (2023). https://doi.org/10.1007/s13304-023-01648-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-023-01648-8