Abstract
The objective is to investigate preoperative body mass index (BMI) in patients receiving beyond total mesorectal excision (bTME) surgery. The primary end point is length of postoperative stay. Secondary end points are length of intensive care stay, postoperative morbidity and overall survival. BMI is the most commonly used anthropometric measurement of nutrition and studies have shown that overweight and obese patients can have improved surgical outcomes. Patients who underwent a bTME operation for locally advanced or recurrent rectal cancer were put into three BMI (kg/m2) groups of normal weight (18.5–24.9), overweight (25–29.9) and obese (≥ 30) for analysis. Included are 220 consecutive patients from a single centre. The overall length of stay, in days ± standard deviation (range), for normal weight, overweight and obese patients was 21.14 ± 16.4 (6–99), 15.24 ± 4.3 (7–32) and 19.10 ± 9.8 (8–62) respectively (p = 0.002). The mean ICU length of stay was 5.40 ± 9.1 (1–69), 3.37 ± 2.4 (0–19) and 3.60 ± 2.4 (1–14), respectively (p = 0.030). There was no significant difference between the three groups in terms of postoperative morbidity or overall survival. Patients with a normal weight BMI in this cohort have a significantly longer length of stay in ICU and in hospital than overweight or obese patients. This is seen with no significant difference in morbidity or overall survival.
Similar content being viewed by others
Introduction
Colorectal cancer is the third most common in the world and 30–40% is in the rectum [1, 2]. 10–20% of rectal cancer patients present with locally advanced disease [3, 4]. Around 10% of curative operations for rectal cancer will be locally recurrent [5] and around half of the patients with recurrent rectal cancer have isolated and potentially curable disease [6]. A beyond total mesorectal excision (bTME) operation offers the best chance of cure for locally advanced and recurrent rectal cancer [7, 8].
The incidence of rectal cancer is increasing [9] and survival is improving [10,11,12]. Advancing age, male sex and genetic susceptibility are all associated with a worsening in mortality from rectal cancer [13]. There is a known association between lifestyle factors and the incidence of colorectal cancer; however, the mechanisms are poorly understood [13].
Nutrition in surgery is important as malnutrition and obesity can both impact negatively on outcomes. Hiram et al. in 1936 was the first to report on the importance of nutrition in surgery showing a 33% versus 3.5% mortality rate in peptic ulcer surgery in the malnourished and the well nourished, respectively [14]. A nutritional assessment should consist of ‘a comprehensive approach to diagnosing nutrition problems that uses a combination of the following: medical, nutrition, and medication histories; physical examination; anthropometric measurements; and laboratory data’ [15]. Many tools for nutritional assessment have been suggested, with varying definitions of malnutrition; however, there is no agreement on a gold standard [16, 17]. Measuring a patient’s BMI [18] is the most commonly used anthropometric measurement of nutritional status [19]. There is a large variation of BMI thresholds used in the literature [20, 21] and the use of BMI alone as an indicator of nutrition has limitations including not distinguishing between low weight due to fat depletion or muscle depletion [22].
Malnutrition can be created by the systemic effects of cancer or the hosts response to cancer and can be compounded by chemoradiotherapy [23,24,25,26,27]. Patients with gastrointestinal cancers are high risk for presenting with and developing malnutrition as they can create a catabolic effect and cause anorexia, nausea, vomiting, gastrointestinal tract obstruction and malabsorption [28, 29]. 30–60% of colorectal cancer patients and 80% of advanced colorectal cancer patients are reported as malnourished [27, 30]. Malnutrition impacts on morbidity, mortality, length of stay, readmission rates, quality of life and it is considered to negatively affect all bodily functions [16, 17, 25, 27, 31,32,33,34].
The World Health Organisation states that worldwide obesity has more than doubled since 1980 [35]. In 2015, National Health Service England reported an increase in the prevalence of obesity noting that 58% of women, 65% of men and 20% of 5 and 6 year olds are overweight or obese [36]. Being overweight or obese is associated with an increase in the incidence of multiple cancers [37, 38] and an increase in overall mortality in the general population [39, 40]. It can make an operation more technically difficult and increase postoperative complications [38, 41,42,43]. High BMI specifically is an established risk factor for developing colorectal cancer, obese men of all ages are at the greatest risk [44, 45], and the mechanism is unclear [38, 43]. It may be due to a direct biological mechanism such as central obesity, associated with insulin resistance [42, 46], or the creation of a pro-inflammatory state which, amongst other mechanisms, have been implicated in the development of colorectal cancer [41, 43]. It may also be due to indirect mechanisms such as lifestyle choices of being sedentary, eating the wrong foods or smoking [43].
This work aims to examine the impact that preoperative BMI has on postoperative outcomes in locally advanced and recurrent bTME rectal cancer.
Method
Inclusion criteria
Consecutive adults undergoing a curative intent bTME operation, as defined by the beyond TME consensus statement [4], for locally advanced primary or recurrent rectal adenocarcinoma, under the care of the senior authors at The Royal Marsden Hospital were included. All patients had a preoperative BMI recorded.
End points
The primary end point is the effect that BMI has on in-hospital length of stay. Secondary end points are to assess the effect that BMI has on intensive care length of stay, postoperative morbidity and overall survival.
Definitions
Definition of BMI categories
The World Health Organisation (WHO) BMI categories were used in this work; BMI (kg/m2): ≤ 18.5 as underweight, 18.5–24.9 as normal weight, 25–29.9 as overweight, and ≥ 30 as obese [18].
Definition of operative groups
Pelvic exenteration: a multivisceral resection of pelvic contents to clear central, anterior, posterior, lateral or inferior compartments as is required. bTME other: an operation for a tumour that extends beyond the circumferential resection margin on preoperative imaging.
Data source
Patients were identified from a database at The Royal Marsden Hospital from January 2006 to December 2016. Computerised records for each patient were retrospectively interrogated. Body Mass Index (BMI) (kg/m2) was measured immediately before surgery as part of the preoperative assessment. Clinical outcomes were collected retrospectively from a prospectively kept computerised record of the patient’s admission.
Treatment
Patient evaluation included a history and examination, endoscopy with a biopsy, a computed tomography scan of the thorax, abdomen and pelvis (CT-TAP) and a pelvic MRI scan. If the tumour had high-risk features or if distant metastasis was suspected, a positron emission tomography (PET) scan was performed. Ongoing management plans were agreed through a specialised bTME multi-disciplinary team (MDT) meeting.
Chemoradiotherapy was given according to European Guidelines; radiation of 45–50.4 Grays in 25–30 fractions over 5 weeks with concomitant chemotherapy of 5-fluorouracil (5-FU)/capecitabine. From 2010, onwards patients were also considered for induction chemotherapy. Decisions regarding adjuvant chemotherapy were made at the MDT.
Surgery was undertaken either immediately or 6–8 weeks after neoadjuvant therapy by a Consultant-led team experienced in complex rectal cancer surgery. Where appropriate, Consultant-led teams in Plastic and Reconstructive surgery, Urology, Gynaecology and Vascular surgery were involved. All patients were admitted to the ICU postoperatively as standard and discharge from the ICU was made by an intensive care consultant.
Statistics
All statistical comparisons are between the BMI (kg/m2) groups of normal weight (18.5–24.9), overweight (25–29.9) and obese (≥ 30). The BMI data were tested for normality using Shapiro–Wilk test. To investigate differences between the BMI groups, the analysis of variance (ANOVA) test was used. For survival analysis, the Kaplan–Meier method was used and comparison between the groups was with the log rank Mantel–Cox test for significance. Estimated mean survival is given along with the 95% confidence intervals (95% CI). All statistical analysis was considered significant with a p value of 0.05 or less.
Results
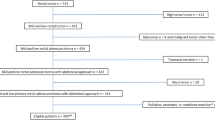
320 patients had undergone a bTME resection, as defined by the bTME consensus statement [4], 52 of which included a sacrectomy under the senior authors. 4 palliative resections, 35 non-adenocarcinoma tumours and 61 patients from outside of our institution were excluded.
Included are 220 consecutive patients. 179 (81.4%) patients received neoadjuvant chemoradiotherapy, 6 (2.7%) received chemotherapy only, 4 (1.8%) received radiotherapy only and 31 (14.1%) had no neoadjuvant therapy. 106 (48.2%) patients had adjuvant therapy. 151 patients underwent a pelvic exenteration and 34 of them had an en bloc sacrectomy. 69 patients had a ‘bTME other’ operation that included 14 recurrent rectal cancers, 14 synchronous resections, 10 with MRI predicted pelvic sidewall involvement, 26 and 5 with involved and threatened circumferential resection margins, respectively.
Follow-up time ranged from 1.5 to 119.6 months with a median follow-up time of 26.0 months. The 3-year disease-free survival is 66% and the 5-year overall survival is 71%.
The BMI data were tested using Shapiro–Wilk test which showed the data to be normally distributed (p < 0.0001). Each BMI category of normal weight, overweight and obese was also tested separately and they are all normally distributed (p = 0.005, p < 0.0001 and p < 0.0001, respectively).
The BMI (kg/m2) categories of normal weight (18.5–24.9), overweight (25–29.9) and obese (≥ 30) had 81, 97 and 42 patients, respectively. The mean BMI for the whole cohort ± standard deviation (± SD) (range) was 26.3 ± 4.3 (18.5–43) kg/m2.
Patient demographics are shown in Table 1. There are 138 (62.7%) males and 82 (37.3%) females. When broken into the normal weight, overweight and obese categories, significantly, more males were overweight and obese (p = 0.004). There was no significant difference between the BMI groups in terms of age (p = 0.933) or ASA grade (p = 0.263).
There was no significant difference between the BMI groups in terms of neoadjuvant treatment received (see Table 2). In the group of 31 patients who went straight to surgery, 17 were normal weight, 11 were overweight and 3 were obese which was significantly (p = 0.048) different. There was no significant difference between the groups in terms of extent of surgery (p = 0.767), 30-day morbidity (p = 0.461), overall morbidity (p = 0.563) or morbidity in terms of Clavien–Dindo classification (see Table 2). There was no 90-day mortality in any patients.
The mean ICU length of stay in days, ± SD (range) was 5.4 ± 9.1 (1–69), 3.37 ± 2.4 (0–19) and 3.6 ± 2.4 (1–14) for normal weight, overweight and obese BMI categories, respectively which was statistically significant (p = 0.030). The overall length of stay was 21.14 ± 16.4 (6–99), 15.24 ± 4.3 (7–32) and 19.1 ± 9.8 (8–62) for normal weight, over weight and obese BMI categories, respectively, which was statistically significant (p = 0.002) (see Table 2).
There was no statistically significant difference between the three BMI groups in patients who presented with a locally advanced primary versus recurrent rectal cancer, the postoperative histopathological T and N staging, the completeness of the resection, the overall number of lymph nodes and the number of cancer positive lymph nodes in the specimen (see Table 2).
Mean overall survival was 67.73 (95% CI 59.88–75.57) months, 95.44 (95% CI 82.88–108.01) months and 68.63 (95% CI 61.43–75.83) months for normal weight, over weight and obese BMI categories, respectively, which was not statistically significant (p = 0.283) (see Table 3; Fig. 1).
Discussion
It is surprising how heterogeneous the literature is on the issue of BMI and surgical outcomes in general which is further complicated by a large variation used in BMI cut offs and definitions [34, 47,48,49,50,51]. There is little written about the relationship between BMI and postoperative outcomes in bTME surgery for locally advanced and recurrent rectal cancer. Mullen et al. [48] conducted a multicentre study with 118,707 non-bariatric, general surgery patients investigating the relationship between BMI and morbidity and mortality. Mullen et al. [48] noted a reverse J-shaped relationship where being underweight (BMI < 18.5 kg/m2) suffered the most morbidity and mortality followed by being morbidly obese (BMI > 40 kg/m2). The least morbidity and mortality were seen in the overweight (18.5–25 kg/m2) and obese (25–30 kg/m2) groups [48]. Other studies have also seen an apparent protective effect of being overweight or obese, as opposed to being underweight or morbidly obese, which has been labelled as ‘the obesity paradox’ [48, 50,51,52]. The obesity paradox has been demonstrated in a variety of areas including colorectal cancer [34, 52, 53], critically ill patients [54], renal failure [55], heart failure [56] coronary artery disease patients undergoing intervention [51] and general surgery patients [48]; however, the reason for the observed effect is not clear.
Burden et al. [32] reported on 87 UK patients with colorectal cancer, who underwent surgery, and the preoperative mean BMI ± SD was 26.6 ± 5.1 kg/m2. Read et al. [57] reported on 51 patients with advanced colorectal cancer who had a median BMI (range) of 27 (17–41) kg/m2. Beaton et al. [52] looked at 31 patients with colorectal cancer undergoing pelvic exenteration with a mean BMI ± SD of 24.3 ± 5.9 kg/m2. The mean BMI in this cohort, ± SD (range) was 26.3 ± 4.3 (18.5–43) kg/m2. McWhirter et al. [22] reported that in a UK hospital, 23% of men and 28% of women admitted for any reason have a BMI of less than 20 kg/m2. In this cohort, 15 (6.8%) patients had a BMI of less than 20 kg/m2. It is interesting to note that this cohort did not see large amounts of low BMI patients, despite all being either locally advanced or recurrent rectal cancer; these data do, however, match up with previously published data on BMI in colorectal cancer [22, 32, 52, 57].
There are significantly more overweight and obese males in this cohort, which would be expected as obesity rates are higher in males across the general United Kingdom population [36]. Furthermore, being male and overweight or obese are risk factors for developing colorectal cancer [37, 38]. This male predominance may be related to central adiposity which is more common in males and is linked with metabolic abnormalities which is thought to be a risk factor for developing colorectal cancer [46].
Morbidity
Morbidity rates after resection for locally advanced and recurrent rectal cancer are expected to be high due to the extent and complexity of the surgery. In bTME surgery, all-cause morbidity is expected to be above 50% [58] and a range between 35–78% is quoted in the literature [53, 58,59,60,61]. It is also expected that morbidity rates after surgery for recurrent rectal cancer would be worse [61]. Moghadamyeghaneh et al. [58] reported that after pelvic exenteration, 65.7% experienced morbidity, with infective (42.6%) and haemorrhagic (39.0%) complications being the two largest causes. Healy et al. [53] reported on 414 patients with colorectal cancer and found no significant difference in the overall postoperative morbidity between BMI groups. Healy et al. [53] reported that patients with a BMI arbitrary cut-off point of less than 20 kg/m2 had significantly more ‘major complications’ defined as pneumonia, acute respiratory distress syndrome, abdominal or pelvic abscesses, organ failure and myocardial infarction [53]. In this cohort, 87 (39.5%) and 110 (50.0%) patients experienced morbidity in the first 30 days or during the whole admission, respectively. This work did not associate being normal weight, overweight or obese was with any significant difference in postoperative morbidity in the first 30 days, during the whole admission or after being categorised into the Clavien–Dindo classification.
Survival
Overall survival in this cohort was not significantly different across the groups of normal weight, overweight and obese, but it is interesting to note that normal weight had the worst overall survival (see Fig. 1). Healy et al. [53] also noted a non-significantly worse survival in the non-obese group across a colorectal cancer cohort [53]. Mullen et al. [48] reported that overweight and obese general surgery patients have a significantly lower crude and adjusted mortality rate compared to normal weight patients [48]. This finding is unexpected and is contrary to the general population where studies have shown that an increased BMI or having an overweight or obese status is linked with increased mortality [39, 40]. The BMI measurements in this study are a single snap shot and will not have accounted for recent weight loss. It has been shown previously that colorectal cancer patients, with a normal BMI, can be malnourished [32, 57]. It is conceivable that patients in the normal BMI range may have recently lost weight and may be in a state of malnourishment.
Length of stay
Mullen et al. [48] reported a significant difference in the mean length of stay between underweight (BMI < 18.5 kg/m2), and obese (BMI 30–35 kg/m2) patients of 9.1 days and 4.1 days, respectively [48]. Beaton et al. [52] reporting on exenterative patients, also noted a significant difference in the mean length of stay of 50 days if underweight (< 18.5 kg/m2), 16.0 days if normal weight (18.5–25 kg/m2) and 14 days if overweight/obese (> 25 kg/m2) [52]. Garth et al. [31] undertook a comprehensive assessment of nutrition in patients with upper gastrointestinal and colorectal cancer and reported a doubling of the length of stay in malnourished patients [31]. These data show a significantly longer length of stay in ICU and overall for patients in the normal weight as compared to overweight or obese categories, without significantly more morbidity which has been shown before [31, 48, 52, 57]. This work is not able to define the causality of this finding; however, a state of malnutrition may be implicated.
Limitations
This is a retrospective single-centre analysis. Patients undergoing bTME surgery are carefully considered which introduces a selection bias. This work looks at the impact that a single variable, measured on a single occasion, has on outcomes that are multifactorial. BMI as a single measure of nutrition is likely to be inadequate in comparison to other multifaceted measurement tools. Further data points on preoperative comorbidities beyond ASA status or intraoperative data points on the complexity or length of surgery and reconstruction would have been useful to examine these endpoints further and should be included in any further work.
Conclusion
The BMI, especially in isolation, as a measure of nutritional status has drawbacks but is commonly used. This work found that normal weight patients have a significantly longer length of ICU and overall hospital stay compared to overweight and obese patients. This was seen despite no significant difference in postoperative morbidity or overall survival. This study adds evidence to the existence of an obesity paradox in bTME rectal cancer but cannot illuminate upon causality.
The message from this work and other studies is contrary to the thinking that overweight or obese colorectal patients are a high surgical risk; in fact, the opposite appears to be true. Further work is required to better define and investigate this area.
References
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS et al (2014) Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 15(11):1245–1253
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Mohan HM, Evans MD, Larkin JO, Beynon J, Winter DC (2013) Multivisceral resection in colorectal cancer: a systematic review. Ann Surg Oncol 20(9):2929–2936
Beyond TMEC (2013) Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 100(8):E1–E33
Faerden AE, Naimy N, Wiik P, Reiertsen O, Weyessa S, Tronnes S et al (2005) Total mesorectal excision for rectal cancer: difference in outcome for low and high rectal cancer. Dis Colon Rectum. 48(12):2224–2231
Wanebo HJ, Antoniuk P, Koness RJ, Levy A, Vezeridis M, Cohen SI et al (1999) Pelvic resection of recurrent rectal cancer: technical considerations and outcomes. Dis Colon Rectum. 42(11):1438–1448
Ferenschild FT, Vermaas M, Verhoef C, Ansink AC, Kirkels WJ, Eggermont AM et al (2009) Total pelvic exenteration for primary and recurrent malignancies. World J Surg. 33(7):1502–1508
Simillis C, Baird DL, Kontovounisios C, Pawa N, Brown G, Rasheed S et al (2017) A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg 265(2):291–299
(2015) National Bowel Cancer Audit Report—2015. This is part of National Bowel Cancer Audit. https://digital.nhs.uk/data-and-information/publications/statistical/national-bowel-cancer-audit/national-bowel-cancer-audit-report-2015. Accessed 16 Feb 2019
Kodeda K, Johansson R, Zar N, Birgisson H, Dahlberg M, Skullman S et al (2015) Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis 17(9):O168–O179
Salerno GV, Daniels IR, Moran BJ, Heald RJ, Thomas K, Brown G (2009) Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum 52(4):632–639
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T et al (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12(6):575–582
Tarraga Lopez PJ, Albero JS, Rodriguez-Montes JA (2014) Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol 7:33–46
Hiram S (1936) Percentage of weight loss: a basic indicator of surgical risk in patients with chronic peptic ulcer. JAMA 6(106):458–460
Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition Board of Directors (2011) A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr 35(1):16–24
Compher C, Higashiguchi T, Yu J, Jensen GL (2017) Does low body mass index predict the hospital mortality of adult Western or Asian patients? JPEN J Parenter Enteral Nutr. 2017:148607117713182
Gupta D, Lis CG, Granick J, Grutsch JF, Vashi PG, Lammersfeld CA (2006) Malnutrition was associated with poor quality of life in colorectal cancer: a retrospective analysis. J Clin Epidemiol 59(7):704–709
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253.
World Health Organisation (1995) Physical status: the use and interpretation of anthropometry Report of a WHO Expert Committee. World Health Organisiation, Geneva
Jensen GL (2016) Global leadership conversation: addressing malnutrition. JPEN J Parenter Enteral Nutr 40(4):455–457
Flegal KM, Kit BK, Graubard BI (2014) Body mass index categories in observational studies of weight and risk of death. Am J Epidemiol 180(3):288–296
McWhirter JP, Pennington CR (1994) Incidence and recognition of malnutrition in hospital. BMJ 308(6934):945–948
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69
Gupta D, Vashi PG, Lammersfeld CA, Braun DP (2011) Role of nutritional status in predicting the length of stay in cancer: a systematic review of the epidemiological literature. Ann Nutr Metab 59(2–4):96–106
Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S (2015) Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J 14:91
Yamano T, Yoshimura M, Kobayashi M, Beppu N, Hamanaka M, Babaya A et al (2016) Malnutrition in rectal cancer patients receiving preoperative chemoradiotherapy is common and associated with treatment tolerability and anastomotic leakage. Int J Colorectal Dis 31(4):877–884
Lopes JP, de Castro Cardoso Pereira PM, dos Reis Baltazar Vicente AF, Bernardo A, de Mesquita MF (2013) Nutritional status assessment in colorectal cancer patients. Nutr Hosp 28(2):412–418
Fettes SB, Davidson HI, Richardson RA, Pennington CR (2002) Nutritional status of elective gastrointestinal surgery patients pre- and post-operatively. Clin Nutr. 21(3):249–254
Nitenberg G, Raynard B (2000) Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. 34(3):137–168
Karthaus M, Frieler F (2004) Eating and drinking at the end of life. Nutritional support for cancer patients in palliative care. Wien Med Wochenschr 154(9–10):192–198
Garth AK, Newsome CM, Simmance N, Crowe TC (2010) Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet 23(4):393–401
Burden ST, Hill J, Shaffer JL, Todd C (2010) Nutritional status of preoperative colorectal cancer patients. J Hum Nutr Diet 23(4):402–407
Waitzberg DL, Correia MI (2003) Nutritional assessment in the hospitalized patient. Curr Opin Clin Nutr Metab Care 6(5):531–538
Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G (2016) Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Tech Coloproctol 20(8):517–535
World Health Organisation (2016) Obesity and overweight. https://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 16 Feb 2019
(2016) National statistics: statistics on obesity, physical activity and giet—England, 2016. https://content.digital.nhs.uk/catalogue/PUB20562. Accessed 16 Feb 2019
Leitzmann MF, Moore SC, Koster A, Harris TB, Park Y, Hollenbeck A et al (2011) Waist circumference as compared with body-mass index in predicting mortality from specific causes. PLoS One 6(4):e18582
Larsson SC, Rutegard J, Bergkvist L, Wolk A (2006) Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer 42(15):2590–2597
Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R et al (2006) Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355(8):763–778
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348(17):1625–1638
Klampfer L (2011) Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets 11(4):451–464
Vigneri PG, Tirro E, Pennisi MS, Massimino M, Stella S, Romano C et al (2015) The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol. 5:230
Shaukat A, Dostal A, Menk J, Church TR (2017) BMI is a risk factor for colorectal cancer mortality. Dig Dis Sci 62:2511–2517
Frezza EE, Wachtel MS, Chiriva-Internati M (2006) Influence of obesity on the risk of developing colon cancer. Gut 55(2):285–291
Kim SE, Shim KN, Jung SA, Yoo K, Moon IH (2007) An association between obesity and the prevalence of colonic adenoma according to age and gender. J Gastroenterol 42(8):616–623
Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A et al (2006) Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst 98(13):920–931
Flegal KE, Lustig C (2016) You can go your own way: effectiveness of participant-driven versus experimenter-driven processing strategies in memory training and transfer. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 23(4):389–417
Mullen JT, Moorman DW, Davenport DL (2009) The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 250(1):166–172
Fonarow GC (2007) The relationship between body mass index and mortality in patients hospitalized with acute decompensated heart failure. Am Heart J 154(5):e21
Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M, Committee ASA et al (2007) An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J 153(1):74–81
Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE et al (2002) The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 39(4):578–584
Beaton J, Carey S, Solomon MJ, Tan KK, Young J (2014) Preoperative body mass index, 30-day postoperative morbidity, length of stay and quality of life in patients undergoing pelvic exenteration surgery for recurrent and locally-advanced rectal cancer. Ann Coloproctol 30(2):83–87
Healy LA, Ryan AM, Sutton E, Younger K, Mehigan B, Stephens R et al (2010) Impact of obesity on surgical and oncological outcomes in the management of colorectal cancer. Int J Colorectal Dis 25(11):1293–1299
O'Brien JM Jr, Phillips GS, Ali NA, Lucarelli M, Marsh CB, Lemeshow S (2006) Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med 34(3):738–744
Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK (1999) Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 55(4):1560–1567
Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F et al (2005) The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 165(1):55–61
Read JA, Choy ST, Beale PJ, Clarke SJ (2006) Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer 55(1):78–85
Moghadamyeghaneh Z, Hwang GS, Hanna MH, Carmichael JC, Mills S, Pigazzi A et al (2015) Surgical site infection impact of pelvic exenteration procedure. J Surg Oncol 112(5):533–537
Jimenez RE, Shoup M, Cohen AM, Paty PB, Guillem J, Wong WD (2003) Contemporary outcomes of total pelvic exenteration in the treatment of colorectal cancer. Dis Colon Rectum 46(12):1619–1625
Speicher PJ, Turley RS, Sloane JL, Mantyh CR, Migaly J (2014) Pelvic exenteration for the treatment of locally advanced colorectal and bladder malignancies in the modern era. J Gastrointest Surg 18(4):782–788
Lee DJ, Sagar PM, Sadadcharam G, Tan KY (2017) Advances in surgical management for locally recurrent rectal cancer: how far have we come? World J Gastroenterol 23(23):4170–4180
Acknowledgements
This study is supported by The Royal Marsden Hospital, The Institute of Cancer Research, Imperial College London and the Biomedical Research Council (BRC).
Funding
Daniel L. H. Baird has a BRC Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or non-financial conflicts of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was formally approved by the Ethics Committee of the institution where it was developed. Animals were not used.
Informed consent
Informed consent is not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Baird, D.L.H., Simillis, C., Pellino, G. et al. The obesity paradox in beyond total mesorectal excision surgery for locally advanced and recurrent rectal cancer. Updates Surg 71, 313–321 (2019). https://doi.org/10.1007/s13304-019-00631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-019-00631-6