Abstract
Introduction
Lifestyle management, including appropriate modifications of nutrition, exercise, and medication behaviors, is essential for optimal glycemic control. The absence of appropriate monitoring methods to validate the lifestyle change may hinder the modification and continuation of behaviors. In this study, we evaluated whether once-weekly glycated albumin (GA) measurement received via a smartphone application could improve glycemia management in patients with type 2 diabetes mellitus by supporting self-review and modification of lifestyle behaviors.
Methods
This open-label, randomized controlled, single-center study in Japan with an 8-week intervention period was conducted in individuals with type 2 diabetes mellitus and HbA1c levels between 7.0 and 9.0% (53‒75 mmol/mol). The intervention was once-weekly home monitoring of GA with a daily self-review of lifestyle behaviors using a smartphone application, in addition to conventional treatment.
Results
A total of 98 participants (72.0% males; age 63.2 ± 11.4 years; HbA1c 7.39 ± 0.39% [57.3 ± 4.3 mmol/mol]) were randomly assigned to the intervention or control group. Significant decreases of the GA and HbA1c levels from the baseline to the last observation day were observed in the intervention group (− 1.71 ± 1.37% [− 39.1 ± 31.3 mmol/mol] and − 0.32 ± 0.32% [− 3.5 ± 3.5 mmol/mol], respectively). Significant decreases of the body weight, waist circumference, and caloric expenditure (p < 0.0001 and p = 0.0003, p = 0.0346, respectively), but not of the caloric intake (p = 0.678), were also observed in the intervention group as compared with the control group.
Conclusions
Self-review of lifestyle behaviors in combination with once-weekly GA home testing received via a smartphone application might potentially benefit glycemic management in people with type 2 diabetes mellitus.
Trial Registration
jRCTs042220048.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Why carry out this study? |
A frequent but minimally invasive glucose-monitoring method is essential for lifestyle modification by people with type 2 diabetes. |
The efficacy of a once-weekly GA home testing for self-reviewing lifestyle behaviors utilizing a smartphone application was investigated. |
What was learned from this study? |
Significant improvements in the GA and HbA1c levels, caloric intake, body weight, and waist circumferences were observed after an 8-week intervention period in the intervention group compared with the control group. |
Once-weekly GA home testing could be a useful tool to support self-reviewing behaviors and could benefit glycemic management in people with type 2 diabetes mellitus. |
Introduction
Glycemic management through appropriate modifications of nutrition, exercise, and medications is of critical importance in preventing complications associated with type 2 diabetes mellitus [1,2,3]. Lifestyle modifications, including intensive lifestyle interventions, have been reported as being effective for improving glycemic management [4,5,6]. However, guidance on lifestyle improvements provided in hospitals might not be sufficiently complied with by the patients at home. While providing additional intensive therapeutic modification of nutrition and daily exercise might be effective for improving glycemic management, it would significantly require the resources of medical professionals. A home monitoring method that detects behavioral modifications could encourage the patients and make the behavior modification and continuation easier. Such a self-management approach could improve glycemia. Hence, a home glucose-monitoring technology that could support patient-driven behavior modification to improve lifestyles has received increasing attention [7].
Structured self-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM) are known to be effective for glycemic management [8,9,10]. While these are widely utilized in patients receiving insulin therapy, they are not sufficiently well utilized in non-insulin users in Japan and some other countries. The invasiveness and burden of frequent finger-pricking for SMBG and subcutaneous microfiber implantation with the need for periodic replacement of the CGM sensor hinder the widespread use of these glucose-monitoring techniques. Further, non-structural or low-frequency SMBG has been reported as being ineffective for glycemic management [11]. At present, HbA1c monitoring is the most commonly used test for glycemia management in people with type 2 diabetes who are not under insulin therapy.
Patients with type 2 diabetes in Japan visit the hospital once every month or every few months to measurement of the HbA1c level [12, 13]. More frequent testing of HbA1c has been reported as not being effective in improving the glycemia status [14] because of the relatively long half-life of hemoglobin. HbA1c level reflects the average blood glucose levels over the previous approximately 1‒2 months [15], so that any changes in the level only slightly reflect behavior changes in the most recent weeks. This could limit the opportunity for patients to reflect on the effects of their lifestyles on the HbA1c levels and improve their diet and exercise behaviors.
Under these circumstances, we sought a minimally invasive home test that could provide reliable feedback for modifications of diet, exercise, and medication behaviors at an appropriate frequency, and focused on glycated albumin (GA). GA and fructosamine are used as surrogate biomarkers for average blood glucose levels over the previous 2‒3 weeks [16, 17]. Among them, GA measurement can provide more reliable results because it represents a ratio of glycated albumin to the total albumin and does not depend on albumin or globulin concentration [18, 19]. Therefore, GA has been used in individuals with unreliable HbA1c values owing to comorbidities, including chronic kidney disease, hemolytic anemia, and pregnancy [17, 20, 21]. Another advantage of GA, as well as fructosamine, is its relatively rapid change compared with HbA1c. The half-life of albumin is approximately 17 days, which is shorter than that of HbA1c [15]. Consequently, GA measurement could be a novel method for weekly or fortnightly testing of average glucose levels. A recent study indicated that GA correlates better with time-in-range than HbA1c and fructosamine, suggesting that GA could be a superior marker for blood glucose management [19]. It is also related to all-cause mortality and cardiovascular events [22, 23].
We previously studied the effects of biweekly walk-in GA tests without intensive behavior education for people with type 2 diabetes mellitus. While no significant differences in the GA and HbA1c levels were detected, significant suppression of worsening of the body weight, body composition, and eating behavior was observed [24]. The study design was limited by the test method, which requires venous blood collection by a professional and cannot be performed at home. To reduce the burden of frequent hospital visits, we recently developed a method to measure the GA levels using approximately 10-µl blood samples sent via a postal service [25]. In addition, to enhance the effect of the GA monitoring, we developed a smartphone application to return the results of the GA measurements immediately and allow the results to be displayed graphically over time on the subjects’ smartphones. We assumed that the intervention could reduce HbA1c levels by approximately 0.2‒0.6% in 8 weeks. Considering that GA has an approximately four-fold greater variability than HbA1c [26], in addition to its relatively rapid change, GA could change by 0.1‒0.3% per week or more. Therefore, we supposed week-to-week GA change could be visible in the graph and utilized for self-reviewing behaviors. The application also offers an easy-to-use self-reviewing diary of the nutrition, exercise, and medication behaviors to facilitate a weekly evaluation of the behavioral patterns in conjunction with GA outcomes. Thus, we attempted to evaluate the effect of self-reviewing behaviors supported by a once-weekly GA monitoring using the smartphone application, which was added to the standard care, in individuals with type 2 diabetes mellitus.
Methods
Approval of the Study
The Fujita Health University Certified Review Board (CRB4180003) approved the study protocol and the informed consent form. This study was conducted in accordance with the Japanese Clinical Trials Act (Act No. 16 of April 14, 2017), the ethical principles of the Declaration of Helsinki, and all applicable laws and guidelines in Japan. The study was registered at the Japan Registry of Clinical Trials (https://jrct.niph.go.jp/, jRCTs042220048).
Study Participants
We conducted this randomized controlled, open-label study at a single site in Japan. People with type 2 diabetes mellitus were enrolled at the Jinnouchi Hospital Diabetes Care Center, Kumamoto, Japan. Participants were required to:
-
1.
Meet the diagnostic criteria for type 2 diabetes mellitus of the Japan Diabetes Society;
-
2.
Have an HbA1c level measured on the date of consent of between 7.0% (53 mmol/mol) and 9.0% (75 mmol/mol);
-
3.
Understand the consent document describing the purpose and methods of the study and agree to participate;
-
4.
Be ≥ 20 years of age at the time of providing consent;
-
5.
Have a smartphone that can run the application.
The exclusion criteria are described in Supplementary Table S1. A research nurse prescreened all patients who visited the hospital within the research period, referring to their medical records. Potentially eligible individuals were requested for a face-to-face assessment to confirm their eligibility (visit 1). All participants provided written informed consent.
Randomization
Before the start of the study, a randomization list was computer-generated by pseudo-random number generation. A permuted block randomization with a block size of 2 was used. To ensure comparability between the two groups, the study participants were stratified by the HbA1c level (< 7.5% and ≥ 7.5% [59 mmol/mol]) at their first visit. Using the randomization list, investigators assigned groups (intervention or control group at a 1:1 ratio) to the participants in numerical order of acceptance.
GA Monitoring and Intervention
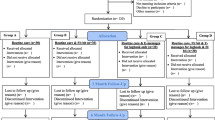
At the start of the study, all participants were informed of the objective of this study and that GA is a surrogate indicator for HbA1c or average blood glucose levels. Participants were randomly assigned to the intervention or control group at the first visit (week − 1). Those assigned to the intervention group were requested to collect finger-prick blood samples once a week and send them to the GA measuring laboratory. GA levels were typically reported the next day and no later than 4 days after the blood collection. The results of the GA measurement were returned to each participant’s GA monitoring phone application, which provided a graphic display of the changes in the GA levels over time. As a part of the initial instruction on the application usage, participants were guided to focus on the GA-level changes instead of the absolute value. In addition, the participants assigned to the intervention group were asked to self-review their nutrition, exercise, and medication behavior goals daily by choosing from three options, namely, “perfect,” “usual,” and “not achieved” for nutrition and exercise, and “all taken,” “partially taken,” and “not taken” for medication. Participants in the intervention group were also asked to enter their self-determined goals and text comments. The inputs were recorded and displayed on the application. These inputs were designed for participant’s self-reviewing behaviors and were not subject to analysis. Medical personnel could also refer to the GA results and other inputs. The monitoring scheme is illustrated in Fig. 1. All participants assigned to the intervention and control groups received conventional medical care and treatment from the diabetes specialist team at the Diabetes Care Center. Although there were no restrictions on concomitant treatments, study participation was discontinued if any of the exclusion criteria were met. Supplementary Fig. S1 shows the outline of the study procedure. Education for nutrition, exercise, and medication had been provided as a part of the regular medical treatment; however, no additional education was provided for the intervention and control groups. As an initial instruction, guidance on the installation and usage of the phone application was provided to the participants in the intervention group. Additional inquiries regarding application usage were answered via text messaging or phone calls from the participants. Except for answering questions from the participants, the research coordinator did not contact them. The HbA1c levels, body weight, BMI, and waist circumference results were provided to participants in the control and intervention groups as part of regular medical treatment, whereas GA levels of venous blood were not informed.
Endpoints and Assessment
The primary endpoints were the changes in the GA levels, caloric intake, and caloric expenditure. The secondary endpoints were the changes in the HbA1c levels, body weight, BMI, and waist circumference.
The GA levels were measured by two methods—GA measurement in self-sampled finger-prick blood specimens posted to the laboratory in the intervention group [25], and GA measurement by the routine method in venous blood samples collected in the hospital laboratory at the first (baseline), third (interim, approximately 1 month later), and fourth visits (last observation date, approximately 2 months later) in all the participants (both groups). The GA levels measured using the conventional method in venous blood samples were compared between the intervention and control groups. The GA levels measured in the finger-prick blood specimens were sent to the smartphones of each of the individuals of the intervention group to allow the changes in the GA levels over time to be displayed in the app in their smartphone app; they were also referred to by the physician on the third visit to provide feedback.
The caloric intake was calculated using Excel Eiyo-kun version 9 (Kenpakusha, Tokyo, Japan), which measures the amounts of ingredients based on the standard tables for food composition in Japan [27]. The participants responded to the paper-based food intake questionnaire at the first (baseline) and fourth (last) visits containing questions about their daily or weekly food intakes and their amounts. Caloric expenditure was surveyed and calculated using the International Physical Activity Questionnaire [28]. All participants responded to the paper-based Japanese short version of the questionnaire at their first and fourth visits, and the caloric expenditure was calculated using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA). Waist circumference was measured at the level of the umbilicus using a measuring tape with the participant standing with his/her arms hanging relaxed on the sides.
We also collected the following data on adverse events and device deficiency information for this study: any diseases, symptoms, disabilities, infections, deaths, or abnormal laboratory values suspected as being attributable to this study as adverse events, and any application performance-related problems, such as malfunctioning of the GA monitoring application or display problems. Furthermore, at the fourth (last) visit, the participants in the intervention group were asked to rate the usefulness of the smartphone application on a five-point Likert scale (“useful,” “somewhat useful,” “neither useful nor not useful,” “somewhat not useful,” and “not useful”).
Quality Control of the Measurement
HbA1c level was measured using a glycated hemoglobin analyzer (HA-8182, Arkray, Kyoto, Japan). The accuracy of the measurement was controlled according to the manufacturer’s instructions. Internal accuracy control was performed once daily; External accuracy control was performed using tests of the Japanese Association of Medical Technologists once yearly. Glycated albumin levels of venous blood were measured by a clinical laboratory testing company (SRL, Kumamoto, Japan). Glycated albumin levels of fingertip blood were measured as previously described [25]. Internal accuracy control was performed once daily using internally prepared standard serum, in which GA levels were determined using standard materials (JCCRM611, Reference Material Institute for Clinical Chemistry Standards, Yokohama, Japan).
Sample Size and Statistical Analysis
A total of 100 patients, 50 in each group, were planned to be evaluated within the study period at the institution. No prior statistical power analysis was performed because this was a phase II pilot study.
The main comparison was performed using a two-tailed test without correspondence. The null hypothesis was that “the mean change (or rate of change) in the measured values in the intervention and control groups would be equal.” The alternative hypothesis was that “the mean change in the measured values in the intervention and control groups would be different.” The t test was used except for the comparisons described below. The median test was applied to compare the caloric intake and expenditure according to the guideline [28]. The significance level was set at p < 0.05. Statistical analysis was performed using JMP 17.0.0 (JMP Statistical Discovery LLC, Cary, NC, USA).
We analyzed two types of populations. The full analysis set population was defined as all randomly assigned participants, except those who were not eligible for the study and those without measurements of the post-baseline efficacy. The per-protocol set was defined as all participants excluding cases of serious deviations from the study protocol and cases from the full analysis set in which the study was discontinued.
Results
Study Population and Analysis Set
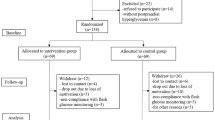
The enrollment began on August 9, 2022, and all visits were completed on January 7, 2023. All patients who visited the hospital within the study period were prescreened. Of those that were, 420 were potentially eligible for screening, and eventually, 99 patients met the eligibility criteria and agreed to participate in this research. Of these 99 individuals, 98 were randomly assigned to the intervention group (n = 48) or control group (n = 50) (Fig. 2). The patient demographics and baseline characteristics of the full analysis set, which were well balanced between the two groups, are presented in Table 1. The race and ethnicity of the participants were not specified, but all the subjects were likely East-Asian.
Flowchart of the study population. FAS indicates the full analysis set, a population defined as all randomly assigned participants, except those who were not eligible for the study and those without measurements of the post-baseline efficacy. PPS indicates the per-protocol set, defined as all participants excluding cases of serious deviations from the study protocol and cases from the full analysis set in whom the study was discontinued
Primary Endpoints
The reduction of the mean GA level from the baseline to the third visit (1 month later) was − 1.03 ± 1.05% (− 23.4 ± 23.9 mmol/mol) in the intervention group and − 0.24 ± 0.85% (− 5.5 ± 19.3 mmol/mol) in the control group. The reduction from baseline to the fourth visit (in 2 months) was − 1.71 ± 1.37% (− 39.1 ± 31.3 mmol/mol) in the intervention group and − 0.05 ± 1.15% (− 1.1 ± 26.2 mmol/mol) in the control group. Both of the reductions were significant (t test, p < 0.0001) (Fig. 3a). The GA results were also consistent between the full analysis set and per-protocol set (Supplementary Tables S2 and S3). Figure 3b shows the mean GA levels measured in the finger-prick blood specimens sampled by the participants themselves of the intervention group. A gradual weekly decrease of the mean GA level was observed.
Changes in the GA and HbA1c levels, body weight, and waist circumference. Changes (with SD) in the GA level (a), HbA1c level (c), body weight (d), and waist circumference (e) from the baseline (first visit) to approximately 1 month (third visit) and 2 months (fourth visit) later. Weekly GA test results in the intervention group are also shown (b). The blue circles and lines indicate the results for the intervention group, and the black squares with broken lines indicate the results for the control group
The mean change in caloric intake from the baseline to the last observation day was − 226 ± 429 kcal in the intervention group and − 164 ± 399 kcal in the control group (Table 2). While the mean change was greater in the intervention group than that in the control group, this difference was not statistically significant (median test, p = 0.6780). The mean change in caloric expenditure from the baseline to the last observation day was significantly higher in the intervention group (729 ± 1856 kcal) than in the control group (− 395 ± 2728 kcal) (median test, p = 0.0346) (Table 2).
Secondary Endpoints
The reduction in mean HbA1c from baseline to the fourth visit (2 months later) was − 0.32 ± 0.32% (− 3.5 ± 3.5 mmol/mol) in the intervention group and 0.03 ± 0.34% (0.3 ± 3.7 mmol/mol) in the control group, being statistically significantly greater in the intervention group (t test, p < 0.0001) (Fig. 3c). Participants in the intervention group also showed a significant decrease of body weight from baseline (− 0.89 ± 0.92 kg) as compared with the control group (− 0.11 ± 0.88 kg; t test, p < 0.0001) (Fig. 3d). The intervention group also showed significant reductions of the BMI and waist circumference (t test, p < 0.0001 and p = 0.0003, respectively) (Fig. 3e, Supplementary Fig. 2).
Adherence to Each Therapy
Adherence to antidiabetic medication was high in both the groups throughout the study (≥ 97%, Supplementary Table 3). The adherence rates to therapeutic modifications of nutrition and exercise are shown in Supplementary Table 4 and Supplementary Table 5. Only a subset of the participants had specific and individualized behavioral goals at the first visit (baseline). The initial adherence rates to therapeutic modifications of nutrition and exercise at the first visit were 96.6 and 82.2%, respectively. Although they were high among individuals who had already established behavioral goals, some individuals did not have specific behavioral goals. During the interviews at visits 1 and 2, these goals were reviewed and added as needed. As a result, the average number of behavioral goals related to therapeutic modifications of nutrition and exercise increased to 2.8 and 2.0 per participant, respectively. Therefore, at the last visit, the degree of achievement of all the behavioral goals, including the goals added at visit 1 or 2, was evaluated using the following four categories: (1) newly achieved goals (achieved): 100% of target behaviors achieved; (2) goals already achieved at the baseline were maintained (maintained); (3) goals not achieved, but adherence improved; the goal actions were not fully (100%) implemented, but were implemented to some extent; (4) not achieved. On the last observation day, a total of 103 out of 124 goals of therapeutic nutrition modification (83.0%) were “achieved” or “maintained”, and a total of 64 out of 88 goals of therapeutic exercise modification (72.7%) were “achieved” or “maintained.”
Assessment of Usefulness
In the subjective assessment of the usefulness of the intervention, namely, the response to the question of “whether weekly GA monitoring was effective in changing daily behaviors, such as those related to diet, exercise, and medication to improve diabetes control” asked at the last visit, “useful” and “somewhat useful” accounted for 97.7% of the responses.
Adverse Events
One participant in the intervention group experienced “medical device site pain” once after finger-prick blood sampling, which resolved 8 days after the onset, with recovery confirmed at 39 days after onset. No other adverse events were related to the intervention were encountered in the course of this study.
Device Deficiencies
Nine failures were reported in seven participants. There were two cases each of “application problems” and “improper user interface,” one case of “equipment difficult to set up or prepare,” three cases of “improper or incorrect procedures or methods” adopted by the user, and one case of “failure to send records.” All the problems were addressed and resolved without harming the participants.
Discussion
In this randomized, open-label study conducted on people with type 2 diabetes mellitus, we investigated the add-on effect of an intervention comprising daily self-review of lifestyle behaviors with once-weekly GA monitoring. The intervention was associated with significantly greater reductions of the GA and HbA1c levels, body weight, and waist circumference as compared with those in the control group (Fig. 3), suggesting that once-weekly GA monitoring, along with self-review of lifestyle behaviors, is an effective tool for supporting self-management of therapeutic modifications of nutrition and exercise, as well as of medication therapy at home.
An average decrease of the HbA1c level of 0.32% (3.5 mmol/mol) over the 8-week study period was observed in the intervention group in this study. This effect appeared to be comparable to the result (average decrease of 0.37%; 4.0 mmol/mol) obtained in subjects with type 2 diabetes mellitus receiving intensive interventions reported from a meta-analysis of 16 trials with intervention periods ranging from 24 weeks to 8 years [29] or another meta-analysis of seven studies based on a 3‒6 month short-term effect of smartphone applications for lifestyle modification targeting people with type 2 diabetes (average decrease of 0.48%; 5.2 mmol/mol or the publication-bias corrected average decrease of 0.30%; 3.2 mmol/mol) [30]. Furthermore, the average reduction in the BMI of 0.58 kg/m2 in our study was more significant than that of 0.29 kg/m2 reported in the aforementioned meta-analysis [29].
In the trials included in the former meta-analysis of the lifestyle intervention studies mentioned above [29], professionals provided educational programs, counseling, supervised exercise therapy, and intensified diet therapy to the participants over relatively long periods. Most studies included in the latter meta-analysis of mobile apps for lifestyle modification [30] requested the participants’ frequent monitoring of blood glucose and recordings of physical activities and dietary details. In contrast, in our study, the burden of monitoring was minimized to once-weekly self-blood sampling, and the lifestyle management was mainly performed in a patient-driven manner, with no additional intensive interventions provided. Participants were screened from patients regularly attending the hospital who had received basic education. All participants were further informed of the purpose of the study at the time of screening, including what the level of GA indicates. Participants in the intervention group were encouraged to input their diet and physical activity goals, self-review their daily behaviors using a smartphone application, and self-review their weekly achievements in a simplistic method by referring to the GA levels sent through the application. Our results suggest that self-review of lifestyle behaviors with once-weekly GA monitoring can improve therapeutic lifestyle modifications and diabetes-related parameters without burdening the participants.
The results of this study must be compared with another study of self-management utilizing a monitoring tool. Our results were similar or slightly inferior to those reported from the previous study of patient-driven lifestyle management using CGM as a tool for monitoring postprandial elevation of the blood glucose (risk-adjusted difference of HbA1c: − 0.50% and body weight difference: − 1.5 kg) [31]. Despite the difficulties in comparing these studies of different designs, the comparison suggests that once-weekly GA monitoring could allow lifestyle modification to a level close to that obtained with CGM with monitoring of the glucose levels after each meal.
No confounding factor analysis was planned in this study because confounding factors for GA had been surveyed [32]. BMI is an established factor that negatively correlates with GA levels [32,33,34]. Body weight and waist circumference are strongly correlated factors of BMI. The negative correlation of BMI to GA levels suggests that the decrease in BMI due to behavioral modification could diminish the decrease in GA levels. Despite this potential negative impact of BMI decrease, the intervention resulted in a significant decrease in the GA levels as well as a significant decrease in body weight, BMI, and waist circumference (Fig. 3 and Supplementary Figure S2). This result is unsurprising because the correlation coefficients of GA and BMI in people with diabetes have been reported to be only 0.2‒0.3 [33, 34]. Hence, it is plausible that the reduction in blood glucose level could potentially outweigh the adverse impact of the decrease in body weight, BMI, and waist circumference.
Our study was initially designed to evaluate the changes in the implementation rate of therapeutic modifications of nutrition, exercise, and medication behaviors and the resulting changes in diabetes-related parameters. This study design implicitly assumed that most patients already had specific and individualized behavioral goals through conventional clinical practice, although they were not effectively achieved. Thus, we tried to determine the efficacy of self-review of lifestyle behaviors supported by once-weekly GA testing. However, contrary to our expectations, our initial interviews revealed that some participants actually did not have specific nutrition and exercise goals. At the end of this interview, most of the participants in the intervention group added goals (Supplementary Tables S5 and S6). Therefore, this study also included the effect of specific and individualized goal setting for therapeutic nutrition and exercise modifications, in addition to the initially intended daily self-review of lifestyle behaviors with once-weekly GA monitoring. This discrepancy between the study design and the actual situation warrants interpretation of the results with caution.
Another limitation of our study is the less-than-optimal reliability of behavioral surveys. We hypothesized that this intervention would improve glucose management through sustained behavioral changes. Therefore, we compared the behaviors in the intervention and control groups by evaluating the caloric intake and expenditure using questionnaires. The caloric intake in the intervention group was significantly lower on the last observation day, which might reflect implementation of the goals of therapeutic modification of diet behaviors. Although the intervention group showed a greater decrease in caloric expenditure (− 226 kcal) than the control group (− 164 kcal), there was no significant difference in the change in caloric intake from the baseline between the two groups (Table 2). The large variability could be the reason for the lack of a statistically significant difference. However, a significant increase in caloric expenditure was observed in the intervention group as compared with the control group, as evidenced by the change from the baseline (Table 2), which may represent the implementation rate of the goals of therapeutic exercise modifications. Caloric intake and expenditure were included in this study because they can be assessed numerically by completing a self-assessment. However, the accuracy of these questionnaires is limited, and both have been reported to show large interpatient variability [35, 36]. The reliability of these values is inferior to that of the changes in the GA and HbA1c levels. Considering these limitations, we may modestly conclude that the behavioral changes measured through the questionnaires did not contradict our hypothesis.
This study was performed at a single study site in Japan, and the participants were all likely East-Asian. Self-care behaviors have been reported as being related to the cultural background [37]. In addition, the pathophysiological mechanisms of diabetes and relationships between the blood glucose levels and monitoring indices have been reported to differ among different ethnicities [38, 39]. Therefore, the applicability of our method to other ethnicities or countries still remains to be examined.
In conclusion, self-review of lifestyle behaviors based on the results of once-weekly GA tests could support self-management of glycemia in people with type 2 diabetes at home.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sone H, Tanaka S, Tanaka S, et al. Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: analysis from the Japan Diabetes Complications Study (JDCS). Diabetologia. 2013;56(5):1021–30. https://doi.org/10.1007/s00125-012-2810-z.
Geng T, Zhu K, Lu Q, et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: a cohort study. PLoS Med. 2023;20(1): e1004135. https://doi.org/10.1371/journal.pmed.1004135.
American Diabetes Association Professional Practice C. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S77–110. https://doi.org/10.2337/dc24-S005.
Ratner R, Goldberg R, Haffner S, et al. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the diabetes prevention program. Diabetes Care. 2005;28(4):888–94. https://doi.org/10.2337/diacare.28.4.888.
Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378(9786):129–39. https://doi.org/10.1016/S0140-6736(11)60442-X.
Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):951–64. https://doi.org/10.1016/S2213-8587(17)30327-3.
Ashrafzadeh S, Hamdy O. Patient-driven diabetes care of the future in the technology era. Cell Metab. 2019;29(3):564–75. https://doi.org/10.1016/j.cmet.2018.09.005.
Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668–75. https://doi.org/10.1177/193229681100500320.
Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–7. https://doi.org/10.2337/dc10-1732.
Price DA, Deng Q, Kipnes M, Beck SE. Episodic real-time CGM use in adults with type 2 diabetes: results of a pilot randomized controlled trial. Diabetes Ther. 2021;12(7):2089–99. https://doi.org/10.1007/s13300-021-01086-y.
Polonsky WH, Fisher L. Self-monitoring of blood glucose in noninsulin-using type 2 diabetic patients: right answer, but wrong question: self-monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care. 2013;36(1):179–82. https://doi.org/10.2337/dc12-0731.
Mitri J, Sugiyama T, Tanaka H, Ohsugi M, Gabbay RA. Understanding the quality of diabetes care in Japan: a systematic review of the literature. Diabetol Int. 2022;13(1):41–8. https://doi.org/10.1007/s13340-021-00497-3.
ElSayed NA, Aleppo G, Aroda VR, et al. Comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S49–67. https://doi.org/10.2337/dc23-S004.
Driskell OJ, Holland D, Waldron JL, et al. Reduced testing frequency for glycated hemoglobin, HbA1c, is associated with deteriorating diabetes control. Diabetes Care. 2014;37(10):2731–7. https://doi.org/10.2337/dc14-0297.
Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18(4):440–7. https://doi.org/10.2337/diacare.18.4.440.
Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33(12):2153–63.
Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104. https://doi.org/10.1016/j.cca.2014.03.001.
Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and pitfalls of fructosamine and glycated albumin in the diagnosis and treatment of diabetes. J Diabetes Sci Technol. 2015;9(2):169–76. https://doi.org/10.1177/1932296814567227.
Desouza CV, Rosenstock J, Kohzuma T, Fonseca VA. Glycated albumin correlates with time-in-range better than HbA1c or fructosamine. J Clin Endocrinol Metab. 2023;108(11):e1193–8. https://doi.org/10.1210/clinem/dgad298.
Freitas PAC, Ehlert LR, Camargo JL. Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab. 2017;61(3):296–304. https://doi.org/10.1590/2359-3997000000272.
Zendjabil M. Glycated albumin. Clin Chim Acta. 2020;502:240–4. https://doi.org/10.1016/j.cca.2019.11.007.
Rooney MR, Tang O, Pankow JS, Selvin E. Glycaemic markers and all-cause mortality in older adults with and without diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2021;64(2):339–48. https://doi.org/10.1007/s00125-020-05285-3.
Zhao H, Hu Q, Chen J, et al. Glycated albumin and risk of cardiovascular diseases and mortality in patients with and without dialysis: a meta-analysis. Diabetes Obes Metab. 2023;25(8):2203–17. https://doi.org/10.1111/dom.15097.
Aihara M, Hayashi T, Koizumi C, et al. Bi-weekly glycated albumin measurement was useful to encourage behavioral changes in people with type 2 diabetes mellitus. Diabetes Therapy. 2023. https://doi.org/10.1007/s13300-023-01452-y.
Aihara M, Irie T, Yasukawa K, et al. Development of a high-performance liquid chromatographic glycated albumin assay using finger-prick blood samples. Clin Chim Acta. 2023;542: 117272. https://doi.org/10.1016/j.cca.2023.117272.
Tahara Y, Shima K. Evaluation of error levels in hemoglobin A1c and glycated albumin in type 2 diabetic patients due to inter-individual variability. Diabetes Res Clin Pract. 2010;89(2):115–20. https://doi.org/10.1016/j.diabres.2010.04.007.
Ministry of Education, Culture, Sports, Science and Technology, Japan. Standard tables of food composition in Japan 2015 (Seventh Revised Version) https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm Accessed on April 19, 2024
The IPAQ group. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. November 2005. https://sites.google.com/view/ipaq/score Accessed on August 1, 2022.
Chen L, Pei JH, Kuang J, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis. Metabolism. 2015;64(2):338–47. https://doi.org/10.1016/j.metabol.2014.10.018.
Wu X, Guo X, Zhang Z. The efficacy of mobile phone apps for lifestyle modification in diabetes: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7(1): e12297. https://doi.org/10.2196/12297.
Kim EK, Kwak SH, Jung HS, et al. The effect of a smartphone-based, patient-centered diabetes care system in patients with type 2 diabetes: a randomized, controlled trial for 24 weeks. Diabetes Care. 2019;42(1):3–9. https://doi.org/10.2337/dc17-2197.
Aihara M, Jinnouchi H, Yoshida A, et al. Evaluation of glycated albumin levels in tears and saliva as a marker in patients with diabetes mellitus. Diabetes Res Clin Pract. 2023;199: 110637. https://doi.org/10.1016/j.diabres.2023.110637.
Koga M, Matsumoto S, Saito H, Kasayama S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J. 2006;53(3):387–91. https://doi.org/10.1507/endocrj.k05-137.
Miyashita Y, Nishimura R, Morimoto A, et al. Glycated albumin is low in obese, type 2 diabetic patients. Diabetes Res Clin Pract. 2007;78(1):51–5. https://doi.org/10.1016/j.diabres.2007.02.021.
Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab. 2001;281(5):E891–9. https://doi.org/10.1152/ajpendo.2001.281.5.E891.
Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. https://doi.org/10.1186/1479-5868-8-115.
Chesla CA, Chun KM, Kwan CM. Cultural and family challenges to managing type 2 diabetes in immigrant Chinese Americans. Diabetes Care. 2009;32(10):1812–6. https://doi.org/10.2337/dc09-0278.
Kodama K, Tojjar D, Yamada S, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96. https://doi.org/10.2337/dc12-1235.
Wolffenbuttel BH, Herman WH, Gross JL, et al. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care. 2013;36(10):2931–6. https://doi.org/10.2337/dc12-2711.
Acknowledgements
The authors thank all the participants and staff of the Jinnouchi Hospital involved in the study. We want to thank Dr. Keiko Yasukawa (University of Tokyo), Tomoko Irie, Mitsumi Nishi, Noriko Miyauchi, Misaki Sekimoto, Rieko Shimamura, and Masaki Yoshizawa (Provigate, Inc.) for once-weekly GA measurement, and Kein Takeda (Provigate, Inc.) for illustrations.
Funding
This research was supported by AMED (Japan Agency for Medical Research and Development), grant number 21le0110022h0001. Provigate, Inc., funded the preparation of this manuscript and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hideaki Jinnouchi, Kenji Yachiku, Masakazu Aihara, Naoto Kubota, Koshin Sekimizu; Methodology: Mariko Taniguchi, Daisuke Kurosawa, Kenji Yachiku; formal analysis and investigation: Hideaki Jinnouchi, Akira Yoshida, Mariko Taniguchi, Eisaku Yamauchi, Itsushi Minoura; writing—original draft preparation: Kenji Yachiku, Itsushi Minoura, Koshin Sekimizu; Writing—review and editing: Hideaki Jinnouchi, Masakazu Aihara, Naoto Kubota; Funding acquisition: Koshin Sekimizu; resources: Hideaki Jinnouchi, Koshin Sekimizu; supervision: Hideaki Jinnouchi, Takashi Kadowaki, Toshimasa Yamauchi, Koshin Sekimizu.
Corresponding author
Ethics declarations
Conflict of Interest
Provigate, Inc. sponsored the joint research project. Hideaki Jinnouchi holds stocks in Provigate, Inc. Koshin Sekimizu is the founder of Provigate, Inc. Daisuke Kurosawa, Kenji Yachiku, and Itsushi Minoura are employed by Provigate, Inc. Takashi Kadowaki is an Editorial Board member of Diabetes Therapy. Takashi Kadowaki was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. All other authors declare that they have no relationships or activities to declare that might introduce bias, or be perceived as introducing bias, in their work.
Ethical Approval
The Fujita Health University Certified Review Board (CRB4180003) approved the study protocol and the informed consent form. We conducted this study was conducted in accordance with the Japanese Clinical Trials Act (Act No. 16 of April 14, 2017), the ethical principles of the Helsinki Declaration of 1964, its later amendments, and all applicable laws and guidelines in Japan. The study was registered at the Japan Registry of Clinical Trials (https://jrct.niph.go.jp/, jRCTs042220048).
Additional information
Prior Presentations: This manuscript is based on work that has been previously presented by Kenji Yachiku at ATTD 2024 in Florence, Italy (6–9 March 2024) as a poster entitled ‘Weekly glycated albumin home monitoring improves glycemic control in people with type 2 diabetes: a randomized pilot study’.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jinnouchi, H., Yoshida, A., Taniguchi, M. et al. Efficacy of Self-Review of Lifestyle Behaviors with Once-Weekly Glycated Albumin Measurement in People with Type 2 Diabetes: A Randomized Pilot Study. Diabetes Ther 15, 1561–1575 (2024). https://doi.org/10.1007/s13300-024-01599-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01599-2